Dublin, Dec. 18, 2025 (GLOBE NEWSWIRE) -- The "Biopharmaceutical Process Analytical Technology Market - Global Forecast 2025-2030" has been added to ResearchAndMarkets.com's offering.
Biopharmaceutical process analytical technology is rapidly advancing to meet the increasing complexity in drug development, manufacturing, and regulatory compliance. Senior decision-makers must navigate this dynamic market to guide investments that foster innovation, resilience, and long-term growth.
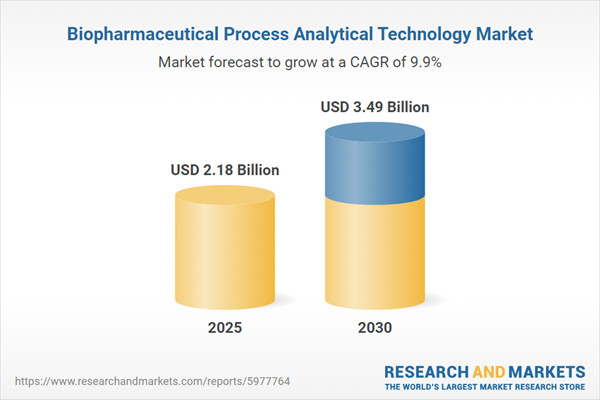
Market Snapshot: Biopharmaceutical Process Analytical Technology Market Size and Growth
The Biopharmaceutical Process Analytical Technology Market expanded from USD 1.98 billion in 2024 to USD 2.18 billion in 2025, experiencing a CAGR of 9.85% and is projected to reach USD 3.49 billion by 2030. This robust growth trajectory positions the technology as vital for enhancing efficiency, regulatory compliance, and competitive positioning in the sector.
Scope & Segmentation
This report provides a comprehensive examination of the market, offering analysis on key segmentation factors, geographic trends, technologies, and strategic landscapes.
- Product Types: Analyzers, sensors & probes (dissolved oxygen, pH, pressure, temperature), software & services.
- Technology: Capillary electrophoresis, chromatography (gas, liquid), particle analysis, and various forms of spectroscopy (mass, NIR, NMR, Raman, UV-visible).
- Measurement Types: At-line, in-line, off-line, and on-line measurement approaches.
- Process Stages: Downstream processing, formulation & fill-finish, upstream processing.
- End Users: Academic research institutes, contract manufacturing organizations, and pharmaceutical & biopharmaceutical companies.
- Applications: Process monitoring, quality control, stability testing, troubleshooting, and root cause analysis.
- Scale: Laboratory bench, micro-scale systems, pilot bioreactors, commercial-scale operations (single-use or stainless-steel equipment).
- Regions:
- Americas: United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru
- Europe, Middle East & Africa: United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya
- Asia-Pacific: China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan
- Companies Analyzed: ABB Ltd., Agilent Technologies, Anton Paar GmbH, Bio-Rad Laboratories, Bruker Corporation, Carl Zeiss AG, Danaher Corporation, Emerson Electric, F. Hoffmann-La Roche AG, GL Sciences Inc., Hamilton Company, Horiba, Jasco, Merck KGaA, Mettler-Toledo, PerkinElmer, Sartorius AG, SCION Instruments, Shimadzu, Siemens, Spectris PLC, SRI Instruments Europe GmbH, Teledyne Technologies, Thermo Fisher Scientific, Waters Corporation.
Key Takeaways for Senior Decision-Makers
- Real-time analytics and hybrid platform integration enhance quality control and reduce production cycle times.
- Advancements in miniaturized biosensors, advanced spectroscopic probes, and digital twins are transforming process monitoring.
- Regulatory trends favor risk-based, continuous verification approaches, promoting early investments in adaptive quality frameworks.
- Collaboration among process engineers, analytical scientists, and data experts enhances process understanding and technology transfer.
- Automation, robotics, and cloud-based infrastructures boost efficiency, enabling remote monitoring and global troubleshooting.
- Partnerships among instrument manufacturers, software vendors, and contract service providers speed up PAT adoption and scale-up.
The Strategic Value of This Report
- Provides segmented market insights critical for evidence-based investment, technology selection, and process improvement initiatives.
- Assists in effective risk management, supply chain planning, and partnership strategies amid regulatory changes and market complexities.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 193 |
| Forecast Period | 2025 - 2030 |
| Estimated Market Value (USD) in 2025 | $2.18 Billion |
| Forecasted Market Value (USD) by 2030 | $3.49 Billion |
| Compound Annual Growth Rate | 9.8% |
| Regions Covered | Global |
Companies Featured
The companies profiled in this Biopharmaceutical Process Analytical Technology market report include:
- ABB Ltd.
- Agilent Technologies, Inc.
- Anton Paar GmbH
- Bio-Rad Laboratories, Inc
- Bruker Corporation
- Carl Zeiss AG
- Danaher Corporation
- Emerson Electric Co.
- F. Hoffmann-La Roche AG
- GL Sciences Inc.
- Hamilton Company
- Horiba, Ltd.
- Jasco, Inc.
- Merck KGaA
- Mettler-Toledo International Inc.
- PerkinElmer, Inc.
- Sartorius AG
- SCION Instruments NL BV
- Shimadzu Corporation
- Siemens AG
- Spectris PLC
- SRI Instruments Europe GmbH
- Teledyne Technologies Incorporated
- Thermo Fisher Scientific, Inc.
- Waters Corporation
For more information about this report visit https://www.researchandmarkets.com/r/uv6i2
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
