Dublin, Dec. 18, 2025 (GLOBE NEWSWIRE) -- The "In Silico Clinical Trials Market - Global Forecast 2025-2032" has been added to ResearchAndMarkets.com's offering.
The in silico clinical trials market is witnessing significant change, fueled by technological innovations and a surge in worldwide investment dedicated to virtual modeling for drug development. These trials offer a strategic advantage for organizations tackling shifting regulatory landscapes and increasing innovation requirements.
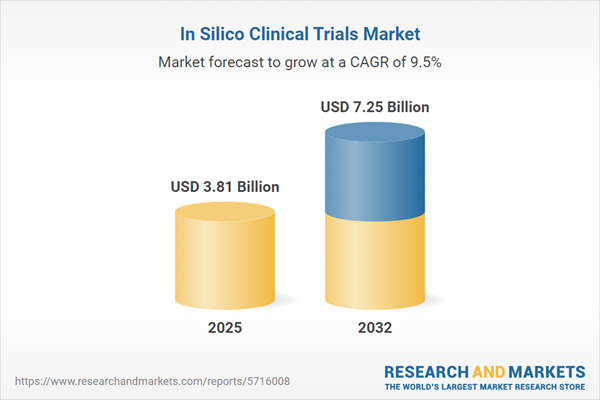
Market Snapshot: In Silico Clinical Trials Market Size and Growth
The market for in silico clinical trials is experiencing a robust growth trajectory, expanding from USD 3.50 billion in 2024 to USD 3.81 billion in 2025, and is projected to soar to USD 7.25 billion by 2032 at a CAGR of 9.51%. The widespread adoption of digital trial technologies, paired with the growing use of real-world data, enables more cost-effective, rapid, and safer methods for preclinical and clinical research on a global scale.
Scope & Segmentation
This report offers a comprehensive analysis and forecasts covering various aspects such as product type, phase, technology platform, application, therapeutic area, end user, and region:
- Product Type: Consulting & Training, Custom Simulation Services, Model Development & Validation, Simulation Software, Trial Design Software, Virtual Patient Modeling
- Phase: Phase I, Phase II, Phase III, Phase IV
- Technology Platform: Artificial Intelligence & Machine Learning, Cloud-Based Simulations, Digital Twin, Mechanistic Modeling, Virtual Patient Population
- Application: Disease Modeling, Drug Development, Medical Device Testing
- Therapeutic Area: Cardiovascular (Arrhythmia Simulation, Atherosclerosis Simulation, Heart Failure Modeling), Infectious Diseases (Parasitic Disease Prediction, Viral Infection Simulation), Neurology (Alzheimer's Simulation, Epilepsy Simulation, Parkinson's Disease Modeling), Oncology (Hematologic Malignancies, Solid Tumors), Rare Diseases (Genetic Disorder Simulation, Orphan Drug Modeling)
- End User: Academic & Research Institutes, Contract Research Organizations, Medical Device Companies, Pharmaceutical & Biotech Companies, Regulatory Agencies
- Region: Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe, Middle East & Africa (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan)
Key Takeaways for Senior Decision-Makers
- In silico clinical trials streamline resource allocation and quicken development timelines, assisting teams in refining study designs and prioritizing candidate therapies prior to human trials.
- Simulations effectively address challenges in rare disease research, ethical trial execution, and complex drug-device interaction assessments, facilitating more informed and secure regulatory submissions.
- The convergence of technologies-such as AI, digital twins, and cloud infrastructure-facilitates more detailed modeling and real-time, multi-site collaboration, broadening opportunities for cross-jurisdictional research.
- Collaborative partnerships are vital for success, allowing organizations to pool expertise, share compliance insights, and develop flexible, modular simulation workflows suitable for any regulatory framework.
- Tariff adjustments, notably in the United States, may impact sourcing, elevate operational costs, and necessitate regionalization of simulation services; risk mitigation demands investment in adaptable technology and robust local partnerships.
- A diverse end-user base, including industry players, academia, and regulatory bodies, underscores the increasing demand for tailored simulation capabilities at every stage of the healthcare innovation process.
Why This Report Matters
- Senior executives gain actionable frameworks for integrating simulations into drug development, enhanced risk management, and quicker market access.
- In-depth segmentation and regional analyses indicate growth opportunities and competitive threats across geographies, technology platforms, and therapeutic applications.
- Strategic recommendations steer investments in talent, partnerships, and infrastructure for sustained digital transformation and regulatory alignment.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 180 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value (USD) in 2025 | $3.81 Billion |
| Forecasted Market Value (USD) by 2032 | $7.25 Billion |
| Compound Annual Growth Rate | 9.5% |
| Regions Covered | Global |
Key Topics Covered:
1. Preface
1.1. Objectives of the Study
1.2. Market Segmentation & Coverage
1.3. Years Considered for the Study
1.4. Currency & Pricing
1.5. Language
1.6. Stakeholders
2. Research Methodology
3. Executive Summary
4. Market Overview
5. Market Insights
5.1. Development of hybrid in silico and real-world evidence platforms to accelerate oncology drug approval processes
5.2. Implementation of regulatory framework alignment for computational trial evidence submission in major global markets
5.3. Integration of mechanistic digital twin simulations for pediatric rare disease drug development
5.4. Adoption of advanced AI-driven pharmacokinetic and pharmacodynamic modeling for personalized virtual patient cohorts
5.5. Leveraging cloud-based high-performance computing to scale virtual clinical trials across geographically diverse populations
6. Cumulative Impact of United States Tariffs 2025
7. Cumulative Impact of Artificial Intelligence 2025
8. In Silico Clinical Trials Market, by Product Type
8.1. Services
8.1.1. Consulting & Training
8.1.2. Custom Simulation Services
8.1.3. Model development & validation
8.2. Software Solutions
8.2.1. Simulation Software
8.2.2. Trial Design Software
8.2.3. Virtual Patient Modeling
9. In Silico Clinical Trials Market, by Phase
9.1. Phase I
9.2. Phase II
9.3. Phase III
9.4. Phase IV
10. In Silico Clinical Trials Market, by Technology Platform
10.1. Artificial Intelligence & Machine Learning
10.2. Cloud-Based Simulations
10.3. Digital Twin
10.4. Mechanistic Modeling
10.5. Virtual Patient Population
11. In Silico Clinical Trials Market, by Application
11.1. Disease Modeling
11.2. Drug Development
11.3. Medical Device Testing
12. In Silico Clinical Trials Market, by Therapeutic Area
12.1. Cardiovascular
12.1.1. Arrhythmia Simulation
12.1.2. Atherosclerosis Simulation
12.1.3. Heart Failure Modeling
12.2. Infectious Diseases
12.2.1. Parasitic Disease Prediction
12.2.2. Viral Infection Simulation
12.3. Neurology
12.3.1. Alzheimer's Simulation
12.3.2. Epilepsy Simulation
12.3.3. Parkinson's Disease Modeling
12.4. Oncology
12.4.1. Hematologic Malignancies
12.4.2. Solid Tumors
12.5. Rare Diseases
12.5.1. Genetic Disorder Simulation
12.5.2. Orphan Drug Modeling
13. In Silico Clinical Trials Market, by End User
13.1. Academic & Research Institutes
13.2. Contract Research Organizations
13.3. Medical Device Companies
13.4. Pharmaceutical & Biotech Companies
13.5. Regulatory Agencies
14. In Silico Clinical Trials Market, by Region
14.1. Americas
14.1.1. North America
14.1.2. Latin America
14.2. Europe, Middle East & Africa
14.2.1. Europe
14.2.2. Middle East
14.2.3. Africa
14.3. Asia-Pacific
15. In Silico Clinical Trials Market, by Group
15.1. ASEAN
15.2. GCC
15.3. European Union
15.4. BRICS
15.5. G7
15.6. NATO
16. In Silico Clinical Trials Market, by Country
16.1. United States
16.2. Canada
16.3. Mexico
16.4. Brazil
16.5. United Kingdom
16.6. Germany
16.7. France
16.8. Russia
16.9. Italy
16.10. Spain
16.11. China
16.12. India
16.13. Japan
16.14. Australia
16.15. South Korea
Companies Featured
The companies profiled in this In Silico Clinical Trials market report include:
- Abzena Ltd.
- Aitia NV
- Certara, Inc.
- Dassault Systemes SE
- Evotec SE
- Exscientia Limited
- GNS Healthcare Inc.
- IBM Corporation
- ICON plc
- Immunetrics Inc.
- Insilico Medicine, Inc.
- InSilicoTrials Technologies SpA
- IQVIA Holdings Inc.
- Novadiscovery SA
- PAREXEL INTERNATIONAL, INC.
- Recursion Pharmaceuticals, Inc.
- Schrodinger, Inc.
- Simulations Plus, Inc.
- The AnyLogic Company
- Virtonomy GmbH
- WuXi AppTec Co., Ltd.
- ZMT Zurich MedTech AG
For more information about this report visit https://www.researchandmarkets.com/r/x6qfp7
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
