Dublin, Nov. 05, 2025 (GLOBE NEWSWIRE) -- The "Europe Wearable Medical Devices Market Analysis and Forecast 2025-2033" has been added to ResearchAndMarkets.com's offering.
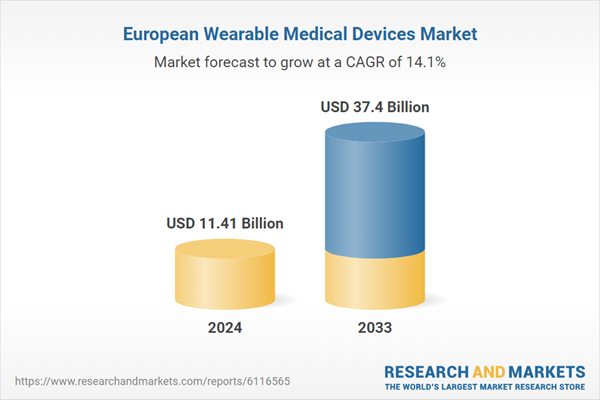
The Europe Wearable Medical Devices Market is anticipated to reach US$ 37.4 billion by 2033 from US$ 11.41 billion in 2024, growing at a CAGR of 14.1% from 2025 to 2033. The market is propelled by rising need for real-time health monitoring, growing incidence of chronic conditions, and evolving wearable technology. Europe's aging population and increasing awareness about health also validate strong market growth.
The major companies profiled in this Europe Wearable Medical Devices market report include:
- Abbott Laboratories
- Activinsights Ltd.
- Fitbit, Inc.
- Garmin Ltd.
- Intelesens Ltd.
- Koninklinje Philips N.V.
- Nuubo
- Omron Corporation
- Polar Electro Oy
Growth Drivers in the Europe Wearable Medical Devices Market
Increasing Incidence of Chronic Diseases
The rising number of chronic diseases like diabetes, cardiovascular diseases, and respiratory diseases is fueling the demand for wearable medical devices in Europe. These technologies facilitate real-time monitoring of health, timely detection of anomalies in health, and remote care, minimizing the frequency of hospital visits. A growing European elderly population further adds to demand since elderly patients are made better off by non-invasive, continuous monitoring.
Wearable devices such as glucose sensors, electrocardiogram patches, and pulse oximeters are used extensively to enhance health outcomes and lower the cost of healthcare, particularly in nations with universal healthcare that seeks to decongest pressure on resources through prevention. January 2023, Over a third of adults across the EU have a chronic disease, and many have multiple morbidities, or two or more chronic diseases. This is especially prevalent among the elderly, with prevalence rates ranging as high as 65% in people aged 65+ and 85% in those aged 85+. As life expectancy continues to grow, the number of people suffering from more than one health issue is projected to increase.
Technology Advances and AI Incorporation
The incorporation of cutting-edge technology including artificial intelligence, machine learning, and Internet of Things (IoT) has greatly improved the functionality and precision of wearable medical devices. AI-based algorithms provide predictive health analytics today, while IoT connectivity provides easy integration with healthcare providers and electronic health records. These are encouraging improved chronic disease control and preventive health interventions.
In Europe, robust R&D infrastructure and digital health programs - such as Germany's DiGA (Digital Health Applications) - foster innovation and adoption. The outcome is a dynamic ecosystem of smart devices designed to address tailored, personalized healthcare needs with little patient effort. March 2022, Infineon Technologies AG and Sleepiz AG released Infineon XENSIV 60 GHz radar technology, embedded in their smart home and medical devices, that has tremendous potential for healthcare uses as they enable precise measurement of vital signs like heartbeat and breathing rate without coming into contact with the body.
Government Support and Regulatory Push
Governments across Europe are taking the leading role in fast-tracking wearable medical technology uptake. Reimbursement policies, funding for telemedicine, and digital health reimbursement models provide a friendly environment for device users and manufacturers. Regulatory agencies like the European Medicines Agency (EMA) and the public healthcare systems are facilitating the approval of digital medical devices. Initiatives like the EU4Health and the European Health Data Space initiative also support the digitalization of healthcare.
These developments fuel trust, adoption, and market growth, particularly as data privacy and device performance are still the major drivers of consumer adoption. Medical devices are regulated within the EU through the Medical Device Regulation (MDR), which entered into effect on April 2017 and into application on May 2021. The necessity for regulation was driven by the desire to create a clear, solid, foreseeable, and enduring regulatory framework.
Challenge in the Europe Wearable Medical Devices Market
Data Privacy and Security Concerns
The most significant challenge in the Europe wearable medical devices market is protecting sensitive patient information. With sensors continuously monitoring and sending health data, there is an increasing risk of data breaches and cyber-attacks. Meeting the General Data Protection Regulation (GDPR) complicates things for manufacturers and healthcare providers as far as handling the data and seeking user consent is concerned.
Maintaining secure data storage, encryption, and appropriate AI use is essential to establishing user trust. Security lapses can result in legal consequences and brand damage, retarding the development of this otherwise lucrative market.
High Cost and Minimal Reimbursement
While wearable medical devices have the potential for significant long-term cost savings and health benefits, device prices and limited insurance reimbursement form adoption barriers. In most European nations, wearable devices are not regarded as reimbursable medical needs but are still viewed as consumer health products.
This disparity impacts patients with lower financial capacities and retards the broader adoption, especially in less affluent parts of Europe. Although some nations are implementing reimbursement programs, variations across borders pose a problem, where it needs harmonized policies and education for healthcare providers to open up accessibility.
Key Attributes
| Report Attribute | Details |
| No. of Pages | 200 |
| Forecast Period | 2024-2033 |
| Estimated Market Value (USD) in 2024 | $11.41 Billion |
| Forecasted Market Value (USD) by 2033 | $37.4 Billion |
| Compound Annual Growth Rate | 14.1% |
| Regions Covered | Europe |
Key Topics Covered
1. Introduction
2. Research & Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. Europe Wearable Medical Devices Market
5.1 Historical Market Trends
5.2 Market Forecast
6. Market Share Analysis
6.1 By Product
6.2 By Site
6.3 By Application
6.4 By Countries
7. Products
7.1 Diagnostic Devices
7.2 Therapeutic Devices
8. Sites
8.1 Strap/Clip/Bracelet
8.2 Headband
8.3 Shoe Sensors
8.4 Handheld
8.5 Others
9. Applications
9.1 Home Healthcare
9.2 Sports and Fitness
9.3 Remote Patient Monitoring
10. Distribution Channels
10.1 Online Distribution
10.2 Retail Pharmacies
10.3 Hypermarkets
10.4 Others
11. Countries
11.1 France
11.2 Germany
11.3 Italy
11.4 Spain
11.5 United Kingdom
11.6 Belgium
11.7 Netherlands
11.8 Russia
11.9 Poland
11.10 Greece
11.11 Norway
11.12 Romania
11.13 Portugal
11.14 Rest of Europe
12. Value Chain Analysis
13. Porter's Five Forces Analysis
13.1 Bargaining Power of Buyers
13.2 Bargaining Power of Suppliers
13.3 Degree of Competition
13.4 Threat of New Entrants
13.5 Threat of Substitutes
14. SWOT Analysis
14.1 Strength
14.2 Weakness
14.3 Opportunity
14.4 Threats
15. Pricing Benchmark Analysis
16. Key Players Analysis
For more information about this report visit https://www.researchandmarkets.com/r/tly7fd
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
