Austin, Oct. 30, 2025 (GLOBE NEWSWIRE) -- Cell And Gene Therapy Clinical Trials Market Size & Growth Analysis:
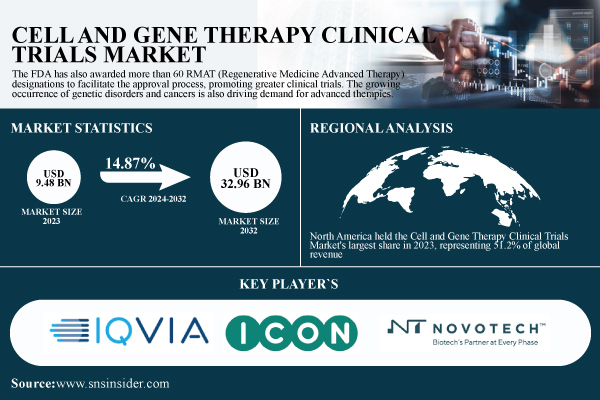
According to a report published by SNS Insider, the global Cell and Gene Therapy Clinical Trials Market was valued at USD 9.48 billion in 2023 and is projected to reach USD 32.96 billion by 2032, expanding at a CAGR of 14.87% during the forecast period 2024–2032.
Market growth is primarily driven by the increasing number of cell and gene therapy candidates entering clinical evaluation, rising investment in regenerative medicine, and favorable regulatory designations such as Breakthrough Therapy and RMAT (Regenerative Medicine Advanced Therapy) status. The growing success of CAR-T and gene-editing therapies in treating hematologic malignancies and rare genetic disorders has also contributed significantly to the market’s rapid expansion.
Get free Sample Report of Cell and Gene Therapy Clinical Trials Market: https://www.snsinsider.com/sample-request/6046
As more biopharmaceutical companies move from discovery to advanced clinical stages, demand for streamlined regulatory processes, patient recruitment strategies, and global trial site networks continues to increase.
Market Overview
The global cell and gene therapy clinical trials market is witnessing remarkable momentum driven by a robust R&D pipeline and regulatory incentives encouraging innovation in personalized medicine. A rising number of biotechnology startups and established pharmaceutical companies are investing heavily in next-generation cell and gene platforms, including CAR-T, TCR-T, AAV, and CRISPR-based therapies.
This dynamic environment is reshaping the clinical research landscape. Increasing emphasis on precision targeting, durable efficacy, and long-term safety monitoring is enhancing the design and conduct of cell and gene therapy clinical studies. Moreover, global harmonization of trial regulations, increased patient access to experimental therapies, and improved manufacturing scalability are accelerating time-to-market for promising therapies.
Segment Analysis
By Phase
Phase II segment was the leader in the Cell and Gene Therapy Clinical Trials Market in 2023, with 58.3% of the total revenue as most of the cell and gene therapy candidates are in mid-stage trials, where safety and efficacy evaluation is essential before advancing into late-stage trials. The most rapidly expanding segment is Phase III, led by a growing number of cell and gene therapy candidates being able to advance successfully beyond mid-stage trials into late-stage clinical confirmation.
By Indication
The Oncology segment was the market leader in the Cell and Gene Therapy Clinical Trials Market in 2023 with a commanding revenue share of 49.7% due to the popularity of CAR-T cell therapies and their growing indications have played a big role in segment leadership. Oncology is the most rapidly growing segment as well, with advances in tumor-targeting cell and gene therapies, growth in patient participation in oncology clinical trials, and growing investment from biotech companies fueling fast growth.
Regional Insights:
North America held the Cell and Gene Therapy Clinical Trials Market's largest share in 2023, representing 51.2% of global revenue. The region’s growth is driven by a mature biotechnology industry, robust regulatory environment, and high investment in cutting-edge therapies.
The Asia-Pacific region is projected to witness the fastest CAGR during the forecast period, driven by an upsurge in clinical trial activity, regulatory modernization, and expanding biotech infrastructure. Countries such as China, Japan, and South Korea are leading this surge, with China emerging as a global hub for cell and gene therapy research. Government incentives, streamlined approval processes, and international partnerships are further boosting the region’s trial activity and global competitiveness.
Need Any Customization Research on Cell and Gene Therapy Clinical Trials Market, Enquire Now: https://www.snsinsider.com/enquiry/6046
Major Players Listed in This Report
- IQVIA – Cell & Gene Therapy Development Solutions
- ICON Plc – ICON Cell & Gene Therapy Services
- Laboratory Corporation of America Holdings (Labcorp Drug Development) – Cell & Gene Therapy Solutions
- Charles River Laboratories International, Inc. – Cell & Gene Therapy Services
- PAREXEL International Corp. – Cell & Gene Therapy Clinical Trial Services
- Syneos Health – Syneos Health Cell & Gene Therapy Solutions
- Medpace Holdings, Inc. – Medpace Cell & Gene Therapy Development Services
- PPD Inc. (part of Thermo Fisher Scientific) – PPD Cell & Gene Therapy Services
- Novotech – Novotech Cell & Gene Therapy CRO Services
- Veristat, LLC – Veristat Cell & Gene Therapy Clinical Development
Recent Developments:
- In March 2025, Amgen and Kyowa Kirin announced that their Phase III IGNITE trial for rocatinlimab in moderate to severe atopic dermatitis (AD) successfully met its co-primary endpoints at 24 weeks. The therapy also achieved all key secondary endpoints, demonstrating statistical significance.
- In March 2025, Neurotech Pharmaceuticals' Encelto (revakinagene taroretcel-lwey/NT-501), an allogeneic encapsulated cell therapy, received FDA approval for macular telangiectasia type 2 (MacTel), making it the first approved treatment for the condition. The therapy is expected to be available in the U.S. by June 2025.
Buy the Cell and Gene Therapy Clinical Trials Market Report Now: https://www.snsinsider.com/checkout/6046
Exclusive Sections of the Report (The USPs):
- CLINICAL TRIAL INCIDENCE & PREVALENCE METRICS – helps you analyze the volume and regional distribution of active cell and gene therapy clinical trials, offering insights into therapeutic focus areas and evolving R&D intensity across key markets.
- SPONSORSHIP & FUNDING LANDSCAPE – helps you understand the investment ecosystem by mapping sponsorship patterns across academia, biotech, and pharma, and tracking regional trends in government, private, and venture capital funding for ongoing and planned trials.
- PATIENT ENROLLMENT & SITE DISTRIBUTION INDEX – helps you identify regional participation levels and trial site density between 2020 and 2032, highlighting regions with optimal recruitment efficiency and infrastructure readiness.
- REGULATORY APPROVAL & SUCCESS RATE ANALYSIS – helps you assess approval patterns and trial success probabilities across geographies, providing clarity on regulatory responsiveness and regional variations in trial outcomes.
- HEALTHCARE INVESTMENT & CAPITAL FLOW INSIGHTS – helps you gauge funding dynamics across government, private, and venture sources in 2023, revealing how financial momentum is shaping innovation pipelines and commercialization timelines.
- GLOBAL TRIAL BENCHMARKING DASHBOARD – helps you compare clinical performance indicators, such as enrollment rates, approval timelines, and success ratios, across leading regions to uncover growth opportunities and partnership hotspots.
Access Complete Report Details of Cell and Gene Therapy Clinical Trials Market Analysis & Outlook: https://www.snsinsider.com/reports/cell-and-gene-therapy-clinical-trials-market-6046
[For more information or need any customization research mail us at info@snsinsider.com]
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
