Dublin, Oct. 23, 2025 (GLOBE NEWSWIRE) -- The "U.S. Clinical Trials Supply and Logistics Market Size, Share & Trends Analysis Report by Service (Logistics & Distribution, Storage & Retention, Packaging, Labeling & Blinding), Phase, Therapeutic Area, End-use, and Growth Forecasts, 2025-2033" report has been added to ResearchAndMarkets.com's offering.
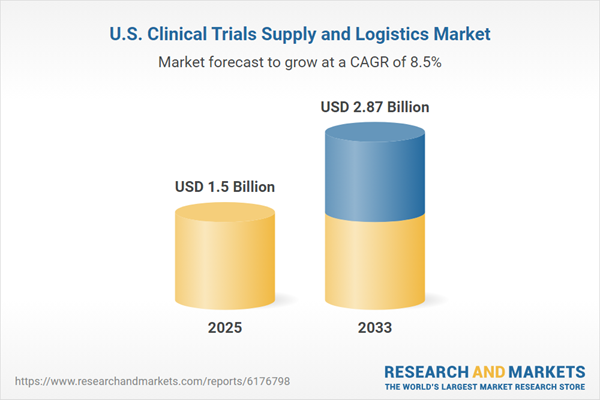
The U.S. clinical trials supply and logistics market size was estimated at USD 1.50 Billion in 2025 and is projected to reach USD 2.87 billion by 2033, growing at a CAGR of 8.45% from 2025 to 2033.
The market has experienced robust growth driven by research and development activities for innovative therapeutic areas such as oncology, rare diseases, and cell and gene therapies.
Besides, the growing complexity of trial designs and regulatory requirements for patient safety and product integrity has led to the need for advanced supply chain models. Furthermore, the adoption of decentralized trials and direct-to-patient delivery solutions has highlighted the significance of flexible and compliant logistics networks. In addition, increased investment from pharmaceutical and biotech companies has enhanced the demand for efficient packaging, temperature-controlled storage, and distribution services essential for timely trial execution.
Moreover, most clinical trials across various phases have been supported by specialized supply solutions such as cold chain distribution, comparator sourcing, clinical packaging, and return management. Smaller batch distributions with tight timelines have been effectively managed for early-phase studies through adaptable supply models, while large-scale Phase III trials benefit from comprehensive global depot networks. Besides, the expanding pipeline of biologics, biosimilars, and advanced therapies has further boosted the need for ultra-low temperature handling and specialized transportation solutions. As trials broaden in scope and geographical reach, supply and logistics providers have become crucial in ensuring the continuous availability of investigational products. Their role has been vital in fueling market expansion by minimizing delays, ensuring compliance, and enhancing trial efficiency.
Furthermore, established market players are pursuing strategic initiatives, including acquisitions, service expansions, and investments in digital platforms to enhance visibility and tracking. Collaborations with sponsors and contract research organizations (CROs) are being accelerated, focusing on increasing cold chain capacity in the U.S. to address the challenges posed by growing trial complexity. Such factors are expected to drive the market over the estimated timeframe.
U.S. Clinical Trials Supply And Logistics Market Report Segmentation
This report forecasts revenue growth at the country level and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, the analyst has segmented the U.S. clinical trials supply & logistics market report based on service, phase, therapeutic area, and end-use.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 120 |
| Forecast Period | 2025 - 2033 |
| Estimated Market Value (USD) in 2025 | $1.5 Billion |
| Forecasted Market Value (USD) by 2033 | $2.87 Billion |
| Compound Annual Growth Rate | 8.4% |
| Regions Covered | United States |
Key Topics Covered:
Chapter 1. Research Methodology and Scope
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.3. Competitive Insights
Chapter 3. U.S. Clinical Trials Supply & Logistics Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.1.1. Technological Advancements in Supply Chain
3.2.1.2. Increasing R&D Investment by Pharmaceutical and Biopharmaceutical Firms
3.2.1.3. Increasing Demand for Outsourcing Services Across the Developing Economies
3.2.1.4. Expansion of Clinical Trial Sites
3.2.2. Market Restraint Analysis
3.2.2.1. Stringent Regulation Pertaining to Logistics
3.2.2.2. Counterfeiting of Drugs
3.2.3. Market Challenges
3.2.4. Market Opportunities
3.3. Technology Landscape
3.4. Pricing Model Analysis
3.5. R&D Spending Analysis (2018-2024)
3.6. Clinical Trial Volume Analysis, 2024
3.7. Tariff Impact Analysis
3.8. Value Chain Analysis
3.9. Market Analysis Tools
3.9.1. Porter's Five Force Analysis
3.9.2. PESTEL by SWOT Analysis
3.9.3. COVID-19 Impact Analysis
Chapter 4. U.S. Clinical Trials Supply & Logistics Market: Service Estimates & Trend Analysis
4.1. U.S. Clinical Trials Supply & Logistics Market, by Service: Segment Dashboard
4.2. U.S. Clinical Trials Supply & Logistics Market, by Service: Movement Analysis
4.3. U.S. Clinical Trials Supply & Logistics Market Estimates & Forecasts, by Service, 2021-2033
4.4. Logistics & Distribution
4.5. Storage & Retention
4.6. Packaging, Labeling, and Blinding
4.7. Manufacturing
4.8. Comparator Sourcing
4.9. Other Services
Chapter 5. U.S. Clinical Trials Supply & Logistics Market: Phase Estimates & Trend Analysis
5.1. U.S. Clinical Trials Supply & Logistics Market, by Phase: Segment Dashboard
5.2. U.S. Clinical Trials Supply & Logistics Market, by Phase: Movement Analysis
5.3. U.S. Clinical Trials Supply & Logistics Market Estimates & Forecasts, by Phase, 2021-2033
5.4. Phase I
5.5. Phase II
5.6. Phase III
5.7. Phase IV
Chapter 6. U.S. Clinical Trials Supply & Logistics Market: Therapeutic Area Estimates & Trend Analysis
6.1. U.S. Clinical Trials Supply & Logistics Market, by Therapeutic Area: Segment Dashboard
6.2. U.S. Clinical Trials Supply & Logistics Market, by Therapeutic Area: Movement Analysis
6.3. U.S. Clinical Trials Supply & Logistics Market Estimates & Forecasts, by Therapeutic Area, 2021-2033
6.4. Oncology
6.5. Cardiovascular Diseases
6.6. Respiratory Diseases
6.7. CNS and Mental Disorders
6.8. Others
Chapter 7. U.S. Clinical Trials Supply & Logistics Market: End Use Estimates & Trend Analysis
7.1. U.S. Clinical Trials Supply & Logistics Market, by End Use: Segment Dashboard
7.2. U.S. Clinical Trials Supply & Logistics Market, by End Use: Movement Analysis
7.3. U.S. Clinical Trials Supply & Logistics Market Estimates & Forecasts, by End Use, 2021-2033
7.4. Pharmaceuticals
7.5. Biologicals
7.6. Medical Device
Chapter 8. Competitive Landscape
8.1. Participant Categorization
8.2. Market Position Analysis, 2024 (Heat Map Analysis)
8.3. Company Profiles
- Thermo Fisher Scientific Inc.
- UPS Healthcare
- Piramal Pharma Solutions
- DHL
- Parexel International
- Almac Group
- UDG Healthcare
- FedEx
- Catalent, Inc.
For more information about this report visit https://www.researchandmarkets.com/r/2m6xhh
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
