Dublin, Oct. 21, 2025 (GLOBE NEWSWIRE) -- The "Pharmaceutical Contract Development and Manufacturing Organization Market Size, Share & Trends Analysis Report by Type (Small Molecule, Large Molecule), Product (API, Drug), Services, Workflow, Therapeutic Area, End Use, and Region with Growth Forecasts, 2025-2033" report has been added to ResearchAndMarkets.com's offering.
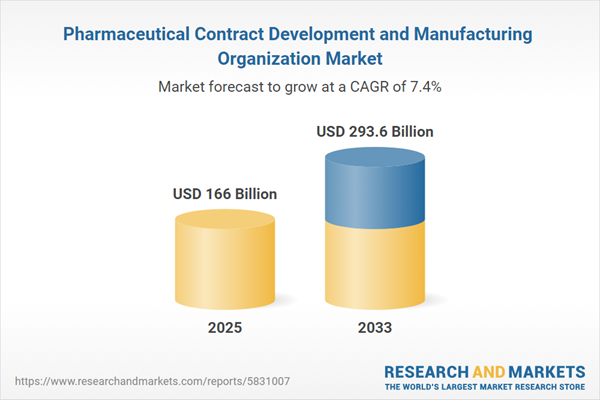
The global pharmaceutical contract development and manufacturing organization market size was estimated at USD 166.0 Billion in 2025 and is projected to reach USD 293.6 billion by 2033, growing at a CAGR of 7.38% from 2025 to 2033. Growth in the market can be attributed to the rising investments by CDMOs to expand new drug development and the increasing demand for novel therapies.
In addition, growing investments in pharmaceutical R&D, rising demand for genetic drugs, and growing prevalence of cancer & age-related disorders, coupled with increasing need for advanced therapeutics, are some of the key factors driving market growth.
The pharmaceutical industry is experiencing a significant shift from traditional small molecules to biopharmaceuticals. This includes monoclonal antibodies, vaccines, recombinant proteins, and other therapies such as mRNA treatments. In addition, this shift is driven by the growing prevalence of chronic diseases, an aging global population, and the increasing access to targeted therapies that tend to provide better efficacy and fewer side effects than conventional treatments.
According to the data published by IQVIA in January 2024, the global medicine spending at list prices is anticipated to rise by 38% between 2024 and 2028, with biologics accounting for more than 40% of that growth, reflecting the central role in the treatments. Regulators such as the U.S. FDA continue to clear record numbers of innovative biologics, approving 50 novel drugs in 2024, of which a significant proportion were advanced biologics and specialty medicines, supported by a 74% first-cycle approval rate that accelerates patient access. Also, biopharmaceuticals represent over half of the late-stage pipeline, and categories such as GLP-1 agonists for diabetes and obesity, cell and gene therapies for rare diseases, and monoclonal antibody-based cancer therapies are shaping demand trajectories. Therefore, CDMOs play an essential role in this environment as biologics production requires complex infrastructure, such as single-use bioreactors, continuous bioprocessing systems, and high-level cold chain logistics, all of which demand expertise and heavy capital investment.
Global Pharmaceutical Contract Development And Manufacturing Organization Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. For this study, the analyst has segmented the global pharmaceutical contract development and manufacturing organization market report based on type, product, service, workflow, therapeutic area, end use, and region.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 250 |
| Forecast Period | 2025 - 2033 |
| Estimated Market Value (USD) in 2025 | $166 Billion |
| Forecasted Market Value (USD) by 2033 | $293.6 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |
Key Topics Covered:
Chapter 1. Research Methodology and Scope
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.3. Competitive Insights
Chapter 3. Pharmaceutical Contract Development and Manufacturing Organization Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.1.1. Growing Consumption of Biopharmaceuticals
3.2.1.2. Increasing Demand for One-Stop-Shop CDMOS
3.2.1.3. Rising Demand for Advanced Therapeutics
3.2.1.4. Increasing Outsourcing Services by Pharmaceutical Companies
3.2.1.5. Upsurge in the Number of Clinical Trials
3.2.2. Market Restraint Analysis
3.2.2.1. Compliance Issues While Outsourcing
3.2.2.2. Changing Scenarios Within Developing Countries
3.2.3. Market Opportunity Analysis
3.3. R&D Spending Analysis
3.3.1. Venture Capital & Government Funding Scenario
3.4. Industry Ecosystem Analysis
3.4.1. Demand Analysis
3.4.2. Supply Chain Analysis
3.5. Technology Landscape
3.6. Clinical Trial Volume Analysis, 2024
3.7. Pricing Analysis
3.8. Tariff and Trade Agreement Impact Analysis
3.9. Value Chain Analysis
3.10. Market Analysis Tools
Chapter 4. Pharmaceutical Contract Development and Manufacturing Organization Market: Type Estimates & Trend Analysis
4.1. Pharmaceutical Contract Development and Manufacturing Organization Market, by Type: Segment Dashboard
4.2. Pharmaceutical Contract Development and Manufacturing Organization Market, by Type: Movement Analysis
4.3. Pharmaceutical Contract Development and Manufacturing Organization Market Estimates & Forecasts, by Type, 2021-2033 (USD Million)
4.4. Small Molecule
4.4.1. Small Molecule Market Estimates and Forecasts, 2021-2033 (USD Million)
4.4.2. Branded
4.4.3. Generic
4.5. Large Molecule
4.5.1. Large Molecule Market Estimates and Forecasts, 2021-2033 (USD Million)
4.5.2. Biologics
4.5.3. Biosimilar
Chapter 5. Pharmaceutical Contract Development and Manufacturing Organization Market: Product Estimates & Trend Analysis
5.1. Pharmaceutical Contract Development and Manufacturing Organization Market, by Product: Segment Dashboard
5.2. Pharmaceutical Contract Development and Manufacturing Organization Market, by Product: Movement Analysis
5.3. Pharmaceutical Contract Development and Manufacturing Organization Market Estimates & Forecasts, by Product, 2021-2033 (USD Million)
5.4. API
5.4.1. API Market Estimates and Forecasts, 2021-2033 (USD Million)
5.4.2. Traditional Active Pharmaceutical Ingredient (Traditional API)
5.4.3. Highly Potent Active Pharmaceutical Ingredient (HP-API)
5.4.4. Biologics
5.4.5. Others
5.5. Drug Product
5.5.1. Drug Product Market Estimates and Forecasts, 2021-2033 (USD Million)
5.5.2. Oral Solid Dose
5.5.3. Semi-Solid Dose
5.5.4. Liquid Dose
5.5.5. Others
Chapter 6. Pharmaceutical Contract Development and Manufacturing Organization Market: Service Estimates & Trend Analysis
6.1. Pharmaceutical Contract Development and Manufacturing Organization Market, by Service: Segment Dashboard
6.2. Pharmaceutical Contract Development and Manufacturing Organization Market, by Service: Movement Analysis
6.3. Pharmaceutical Contract Development and Manufacturing Organization Market Estimates & Forecasts, by Service, 2021-2033 (USD Million)
6.4. Contract Development
6.4.1. Contract Development Market Estimates and Forecasts, 2021-2033 (USD Million)
6.4.2. Pre-formulation & Formulation Development Services
6.4.3. Process Development & Optimization
6.4.4. Analytical Testing & Method Validation
6.4.5. Scale-up & Tech Transfer
6.5. Contract Manufacturing
6.5.1. Contract Manufacturing Market Estimates and Forecasts, 2021-2033 (USD Million)
6.5.2. API Manufacturing
6.5.3. Finished Drug Products Manufacturing
6.6. Packaging and Labelling
6.7. Regulatory Affairs
6.8. Others
Chapter 7. Pharmaceutical Contract Development and Manufacturing Organization Market: Workflow Estimates & Trend Analysis
7.1. Pharmaceutical Contract Development and Manufacturing Organization Market, by Workflow: Segment Dashboard
7.2. Pharmaceutical Contract Development and Manufacturing Organization Market, by Workflow: Movement Analysis
7.3. Pharmaceutical Contract Development and Manufacturing Organization Market Estimates & Forecasts, by Workflow, 2021-2033 (USD Million)
7.4. Clinical
7.5. Commercial
Chapter 8. Pharmaceutical Contract Development and Manufacturing Organization Market: Therapeutic Area Estimates & Trend Analysis
8.1. Pharmaceutical Contract Development and Manufacturing Organization Market, by Therapeutic Area: Segment Dashboard
8.2. Pharmaceutical Contract Development and Manufacturing Organization Market, by Therapeutic Area: Movement Analysis
8.3. Pharmaceutical Contract Development and Manufacturing Organization Market Estimates & Forecasts, by Therapeutic Area, 2021-2033 (USD Million)
8.4. Oncology
8.5. Infectious Diseases
8.6. Neurological Disorders
8.7. Cardiovascular Diseases
8.8. Metabolic Disorders
8.9. Autoimmune Diseases
8.10. Respiratory Diseases
8.11. Ophthalmology
8.12. Gastrointestinal Disorders
8.13. Orthopedic Diseases
8.14. Dental Diseases
8.15. Others
Chapter 9. Pharmaceutical Contract Development and Manufacturing Organization Market: End-Use Estimates & Trend Analysis
9.1. Pharmaceutical Contract Development and Manufacturing Organization Market, by End-Use: Segment Dashboard
9.2. Pharmaceutical Contract Development and Manufacturing Organization Market, by End-Use: Movement Analysis
9.3. Pharmaceutical Contract Development and Manufacturing Organization Market Estimates & Forecasts, by End-Use, 2021-2033 (USD Million)
9.4. Small Pharmaceutical Companies
9.5. Medium Pharmaceutical Companies
9.6. Large Pharmaceutical Companies
Chapter 10. Pharmaceutical Contract Development and Manufacturing Organization Market: Regional Estimates & Trend Analysis
10.1. Regional Market Dashboard
10.2. Regional Market Share Analysis, 2024 & 2033
Chapter 11. Competitive Landscape
11.1. Key Participant Categorization
11.1.1. Market Leaders
11.1.2. Emerging Players
11.2. Market Share/Assessment Analysis, 2024 (Heat Map Analysis)
11.3. Company Profiles
- Thermo Fisher Scientific, Inc
- Lonza
- Recipharm AB
- Catalent, Inc
- WuXi AppTec, Inc
- Samsung Biologics
- Piramal Pharma Solutions
- Siegfried Holding AG
- Corden Pharma International
- Cambrex Corporation
- Vetter Pharma
- Delpharm
- Jubilant Pharmova / HollisterStier
- Eurofins CDMO
- Almac Pharma Services
For more information about this report visit https://www.researchandmarkets.com/r/asbqc1
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
