Dublin, Aug. 11, 2025 (GLOBE NEWSWIRE) -- The "Cell Sheet-based Gene Therapy Market - A Global and Regional Analysis: Focus on Technology Type, Cell-sheet Type, Source Type, Application Type, End User, and Regional Analysis - Analysis and Forecast, 2025-2035" has been added to ResearchAndMarkets.com's offering.
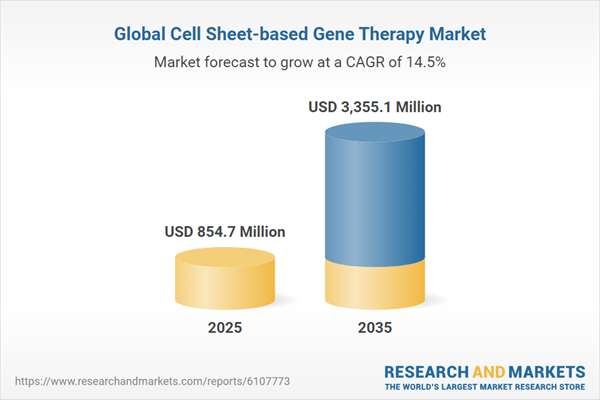
The global cell sheet-based gene therapy market is expected to witness significant expansion, projected to reach $3.33 billion by 2035.
This growth is driven by the rising prevalence of chronic, degenerative, and rare conditions such as limbal stem cell deficiency, ischemic heart disease, and inherited skin disorders that are poorly served by conventional therapies. Cell sheet-based gene therapy offers a regenerative, scaffold-free solution that delivers intact, functional cell layers directly to damaged tissues, enabling superior tissue repair, integration, and localized gene expression.
The cell sheet-based gene therapy market in the Asia-Pacific region is expected to witness substantial growth over the next five years, driven by Japan's progressive regulatory environment, expanding clinical activity, and strong academic-industry collaboration. The region benefits from advanced regenerative medicine frameworks such as Japan's Act on the Safety of Regenerative Medicine and the PMDA's fast-track approval pathways, which have accelerated the development and approval of therapies such as JACC and Holoclar.
Key developments include the late-stage clinical advancement of autologous and allogeneic cartilage cell sheets for osteoarthritis and ongoing efforts in iPS cell-derived retinal therapies. Companies such as CellSeed, J-TEC, and Abeona Therapeutics are actively exploring scalable, clinically viable platforms with a focus on skin, corneal, and cartilage repair. Additionally, public-private initiatives and academic partnerships are fostering translational research, while increasing investment in biomanufacturing and infrastructure is expected to support broader commercialization and access.
Growth is further fueled by supportive global frameworks such as FDA's RMAT designation, EMA's ATMP pathway, and Japan's conditional approval model, which have accelerated the clinical and regulatory trajectory of these therapies. Public investments through NIH (U.S.), AMED (Japan), and Horizon Europe are fostering translational research, while countries such as Italy, the U.S., South Korea, and Germany are scaling GMP-certified infrastructure and manufacturing capacity.
The market is also witnessing technological convergence with innovations in temperature-responsive polymers, gene editing integration, and cryopreservation techniques, enabling more stable and scalable product delivery. While monolayer cell sheets, autologous cell sources, and oncology applications currently dominate, expanding indications in ophthalmology, cardiology, and dermatology signal broad therapeutic relevance.
Despite this momentum, challenges such as high manufacturing costs, limited reimbursement clarity, and fragmented late-stage clinical data remain. However, growing payer confidence, successful real-world outcomes, and cross-sector partnerships are steadily addressing these barriers.
The competitive landscape is led by companies such as Abeona Therapeutics, CellSeed Inc., Japan Tissue Engineering Co. (J-TEC), and Holostem, all of which are advancing first-in-class therapies and building robust commercialization strategies. As healthcare systems increasingly embrace regenerative, precision-based approaches, cell sheet-based gene therapy stands at the intersection of high clinical need and disruptive innovation, with the potential to redefine treatment paradigms across multiple medical disciplines.
Key Market Players and Competition Synopsis
Profiled companies have been selected based on inputs gathered from primary experts, as well as analyzing company coverage, product portfolio, and market penetration.
Key players in the cell sheet-based gene therapy market include leading biotech and regenerative medicine firms such as Abeona Therapeutics, Inc. and Foundation ENEA Tech Biomedical, alongside established players such as CellSeed Inc. and J-TEC. Abeona operates a fully integrated, cGMP-compliant manufacturing facility in Cleveland, Ohio, enabling clinical and commercial-scale production of advanced therapies. Its flagship product, ZEVASKYN (prademagene zamikeracel), is an autologous, gene-corrected cell therapy for recessive dystrophic epidermolysis bullosa (RDEB), demonstrating strong commercial potential in rare dermatological conditions.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 128 |
| Forecast Period | 2025 - 2035 |
| Estimated Market Value (USD) in 2025 | $854.7 Million |
| Forecasted Market Value (USD) by 2035 | $3355.1 Million |
| Compound Annual Growth Rate | 14.4% |
| Regions Covered | Global |
Market Dynamics
Market Drivers
- Advancement in Regenerative Medicine
- Growing Investment in Personalized Medicine
- Technological Advancements in Cell Sheet Therapy
Market Restraints
- Rising Cost of Development and Manufacturing
Market Opportunities
- Substantial Surge in the Rise of Cell Sheet Approaches
- Rising Application for Localized and Minimally Invasive Treatments
Market Challenges
- Regulatory Approval and Ethical Issues
Company Profiles
- Abeona Therapeutics Inc.
- Emmaus Medical, Inc
- CellSeed Inc.
- Fujifilm Cellular Dynamics, Inc. (FCDI) (FUJIFILM Holdings Corporation)
- Japan Tissue Engineering Co. Ltd.
- Foundation ENEA Tech Biomedical
For more information about this report visit https://www.researchandmarkets.com/r/d5shqe
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
