New York, USA, Aug. 07, 2025 (GLOBE NEWSWIRE) -- Cancer Cachexia Clinical Trial Pipeline Shows Potential with Active Contributions from 18+ Key Companies | DelveInsight
The cancer cachexia therapeutics market is gaining traction due to the rising global cancer burden and high prevalence of cachexia in advanced-stage patients. With limited approved treatments, there’s growing demand for therapies that address weight loss, muscle wasting, and appetite loss. Increased awareness, deeper understanding of disease mechanisms, and regulatory support through orphan drug designations are driving R&D. Combined with an aging population and the shift toward holistic oncology care, these factors are expected to fuel sustained market growth.
DelveInsight’s 'Cancer Cachexia Pipeline Insight 2025' report provides comprehensive global coverage of pipeline cancer cachexia therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the cancer cachexia pipeline domain.
Key Takeaways from the Cancer Cachexia Pipeline Report
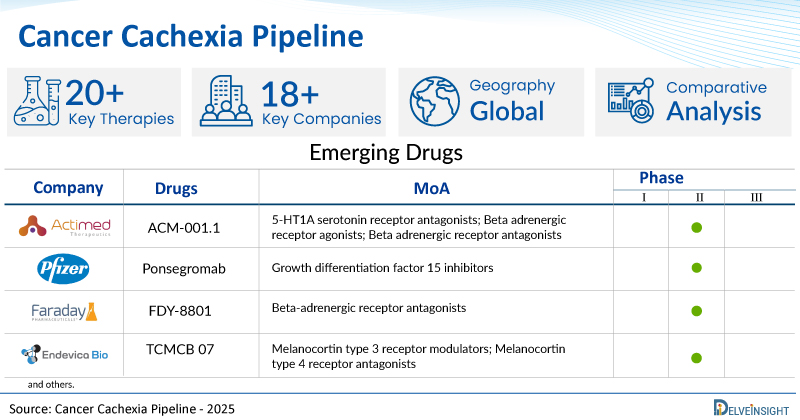
- DelveInsight’s cancer cachexia pipeline report depicts a robust space with 18+ active players working to develop 20+ pipeline cancer cachexia drugs.
- Key cancer cachexia companies such as Changchun GeneScience Pharmaceutical Co., Ltd, Actimed Therapeutics, Pfizer, Faraday Pharmaceuticals, Endevica Bio, Meta Fines, GenFleet Therapeutics, CSPC Pharmaceutical Group Limited, NGM Biopharmaceuticals, AVEO Pharmaceuticals Inc., and others are evaluating new cancer cachexia drugs to improve the treatment landscape.
- Promising pipeline cancer cachexia therapies, such as Nano-crystalline Megestrol Acetate, ACM-001.1, Ponsegromab, FDY-8801, TCMCB 07, ASCA101CC, GFS202A, JMT203, NGM120, AV-380, and others, are in different phases of cancer cachexia clinical trials.
- In April 2025, Endevica Bio announced the dose administration for the first patient in a Phase II trial for its experimental drug TCMCB07 (B07) to prevent weight loss in cancer patients undergoing chemotherapy. In the trial, patients are dosed with B07 as they begin chemotherapy and during the first several rounds of chemotherapy. The primary endpoint is preventing weight loss, which can lead to a debilitating condition called cachexia, a life-threatening wasting syndrome associated with chronic diseases, including cancer.
- In April 2025, the board of directors of CSPC Pharmaceutical Group Limited announced that the antibody drug JMT203, independently developed by Shanghai JMT-BIO Technology Co., Ltd. a subsidiary of the company, has been approved by the US Food and Drug Administration (FDA) to conduct clinical trials in the US. Previously, the Product obtained approval from the National Medical Products Administration of the People’s Republic of China in June 2023 and is currently undergoing clinical trials in China.
- In March 2025, GenFleet Therapeutics announced China’s National Medical Products Administration had approved the clinical trial application for GFS202A in an open-label, multi-center phase I study treating cancer patients with precachexia and cachexia.
- In January 2025, Tensegrity Pharma announced that the company had completed a 150 million yen seed round of financing through a third-party allocation of new shares from Mitsubishi UFJ Capital Limited. This brings the total amount of funds raised to 650 million yen, including 500 million yen from Newton Biocapital Co. The company will use the funds raised through this seed round to accelerate the development of TSP-101, a drug for the treatment of cachexia.
- In January 2025, Meta Fines announced that it has received approval from the Ministry of Food and Drug Safety (MFDS) for its Phase II clinical trial IND for the cancer cachexia treatment, 'ASCA101CC.
- In December 2024, Endevica Bio, announced the start of a Phase II clinical trial of its experimental drug TCMCB07 (B07) to prevent weight loss in certain cancer patients undergoing chemotherapy.
- In February 2024, Meta Fines announced licensing-out agreement with BMI Korea for its cancer cachexia drug ASCA101CC. Under the agreement, BMI Korea will co-own the global technology transfer rights of ASCA101CC with Meta Fines and will have the right to license the product in some countries, including Korea and Southeast Asia.
Request a sample and discover the recent advances in cancer cachexia drugs @ Cancer Cachexia Pipeline Report
The cancer cachexia pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage cancer cachexia drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the cancer cachexia clinical trial landscape.
Cancer Cachexia Overview
Cancer cachexia is a debilitating condition that severely affects patients' quality of life and is linked to poor chemotherapy response and reduced survival rates. It is a widespread problem among individuals with advanced cancer and, as reported by Warren, accounts for around 22% of cancer-related deaths. Characterized as a wasting syndrome, cancer cachexia leads to significant loss of skeletal muscle and fat tissue.
This syndrome not only diminishes quality of life but also limits the patient’s capacity to undergo cancer treatment. Its occurrence differs by cancer type, impacting up to 87% of patients with pancreatic and gastric cancers, but only about 40% in those with breast cancer, sarcoma, leukemia, or Hodgkin lymphoma. As a complication secondary to cancer, cachexia develops alongside systemic inflammation, protein-energy imbalance, and unintentional lean body mass loss. These physiological changes, along with altered body composition, result in noticeable weight loss and disruption of normal biological functions. Early recognition of these symptoms is essential for timely treatment to improve clinical outcomes.
Diagnosing cancer cachexia typically involves observing clinical signs such as unintended weight loss greater than 5% over six months, reduced appetite, declining muscle strength, and deterioration in overall functional capacity. Laboratory tests may show anemia, increased inflammatory markers, and metabolic imbalances, while imaging can reveal tumor burden and complications. It is critical to exclude other possible causes of weight and muscle loss during diagnosis. Once identified, management relies on a multidisciplinary approach involving symptom control, nutritional interventions, physical rehabilitation, and treatment of the underlying cancer, all aimed at enhancing the patient’s well-being and potentially improving treatment success.
Effective management of cancer cachexia requires a personalized, holistic strategy targeting muscle preservation, improving physical condition, and boosting the patient's ability to tolerate cancer therapies. Although the cancer itself continues to progress, cachexia can be mitigated by interrupting the pathways that drive wasting, potentially extending survival. For advanced stages of cachexia, palliative care is the mainstay of treatment. In highly catabolic cancers such as advanced lung or bile duct cancers, the use of agents that inhibit catabolic activity forms the cornerstone of therapy.
Find out more about cancer cachexia drugs @ Cancer Cachexia Treatment
A snapshot of the Pipeline Cancer Cachexia Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| ACM-001.1 | Actimed Therapeutics Ltd | II | 5-HT1A serotonin receptor antagonists; Beta adrenergic receptor agonists; Beta adrenergic receptor antagonists | Oral |
| Ponsegromab | Pfizer | II | Growth differentiation factor 15 inhibitors | Subcutaneous |
| FDY-8801 | Faraday Pharmaceuticals | II | Beta-adrenergic receptor antagonists | NA |
| TCMCB 07 | Endevica Bio | II | Melanocortin type 3 receptor modulators; Melanocortin type 4 receptor antagonists | Subcutaneous |
| GFS202A | GenFleet Therapeutics | II | Growth differentiation factor 15 inhibitors, Interleukin 6 inhibitors | Intravenous |
Learn more about the emerging cancer cachexia therapies @ Cancer Cachexia Clinical Trials
Cancer Cachexia Therapeutics Assessment
The cancer cachexia pipeline report proffers an integral view of the emerging cancer cachexia therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Cancer Cachexia Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Peptides, Polymer, Small molecule, Gene therapy
- Therapeutics Assessment By Mechanism of Action: 5-HT1A serotonin receptor antagonists, Beta adrenergic receptor agonists, Beta adrenergic receptor antagonists, Growth differentiation factor 15 inhibitors, Melanocortin type 3 receptor modulators, Melanocortin type 4 receptor antagonists.
- Key Cancer Cachexia Companies: Changchun GeneScience Pharmaceutical Co., Ltd, Actimed Therapeutics, Pfizer, Faraday Pharmaceuticals, Endevica Bio, Meta Fines, GenFleet Therapeutics, CSPC Pharmaceutical Group Limited, NGM Biopharmaceuticals, AVEO Pharmaceuticals Inc., and others.
- Key Cancer Cachexia Pipeline Therapies: Nano-crystalline Megestrol Acetate, ACM-001.1, Ponsegromab, FDY-8801, TCMCB 07, ASCA101CC, GFS202A, JMT203, NGM120, AV-380, and others.
Dive deep into rich insights for new cancer cachexia treatments, visit @ Cancer Cachexia Drugs
Table of Contents
| 1. | Cancer Cachexia Pipeline Report Introduction |
| 2. | Cancer Cachexia Pipeline Report Executive Summary |
| 3. | Cancer Cachexia Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Cancer Cachexia Clinical Trial Therapeutics |
| 6. | Cancer Cachexia Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Cancer Cachexia Pipeline: Late-Stage Products (Phase III) |
| 8. | Cancer Cachexia Pipeline: Mid-Stage Products (Phase II) |
| 9. | Cancer Cachexia Pipeline: Early-Stage Products (Phase I) |
| 10. | Cancer Cachexia Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Cancer Cachexia Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Cancer Cachexia Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the cancer cachexia pipeline therapeutics, reach out @ Cancer Cachexia Therapeutics
Related Reports
Cancer Cachexia Epidemiology Forecast
Cancer Cachexia Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted cancer cachexia epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Cancer Cachexia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key cancer cachexia companies, including Pfizer, Actimed Therapeutics, Endevica Bio, AVEO Oncology (an LG Chem company), Helsinn Healthcare, Ono Pharmaceutical, among others.
TNF-alpha Inhibitors Market Size, Target Population, Competitive Landscape & Market Forecast – 2034 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key TNF-alpha inhibitors companies, including AbbVie, Amgen, Johnson & Johnson (through Janssen Biotech), UCB, Pfizer, Merck, Samsung Bioepis, Biogen, Takeda Pharmaceutical Company, among others.
Cachexia Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key cachexia companies, including Tvardi Therapeutics, TMS Co. Ltd, RaQualia Pharma, Pfizer, Pephexia Therapeutics, Oncocross, NGM Biopharmaceuticals, MGC Pharmaceuticals, Keros Therapeutics, Incyte Corporation, ImmunoForge, Immuneering Corporation, Green Cross Wellbeing, GlaxoSmithKline, Faraday Pharmaceuticals, Extend Biosciences, Energenesis Biomedica, Endevica Bio, CNBX Pharmaceuticals, CatalYm, Caelus Health, AVEO Oncology, Artelo Biosciences, Aphios Corporation, AliveGen, AEterna Zentaris, Actimed Therapeutics, AAVogen, among others.
Cancer Anorexia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key cancer anorexia companies, including Helsinn Healthcare, Artelo Biosciences, NGM Biopharmaceuticals, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
