Ottawa, Aug. 05, 2025 (GLOBE NEWSWIRE) -- The pharmacovigilance automation market size growth is being driven by rising investments, ongoing innovation, and increasing demand across the healthcare and pharmaceutical sectors, a study published by Towards Healthcare a sister firm of Precedence Research.
Get a free sample to explore key trends, market drivers, and growth insights: https://www.towardshealthcare.com/download-sample/5824
A key reason for this expansion is the tightening of global drug safety regulations and the need to better manage the growing number of adverse drug reactions. To meet these challenges, companies are turning to advanced technologies like artificial intelligence, natural language processing (NLP), and machine learning. These tools are helping automate tasks such as collecting, identifying, and analyzing adverse events making the process faster, more accurate, and cost-effective.
Regionally, North America is leading the market thanks to heavy R&D spending by pharma companies and a strong digital healthcare infrastructure. Meanwhile, Asia Pacific is emerging as the fastest-growing region, driven by a high volume of clinical trials and a robust regulatory landscape.
Key Takeaways
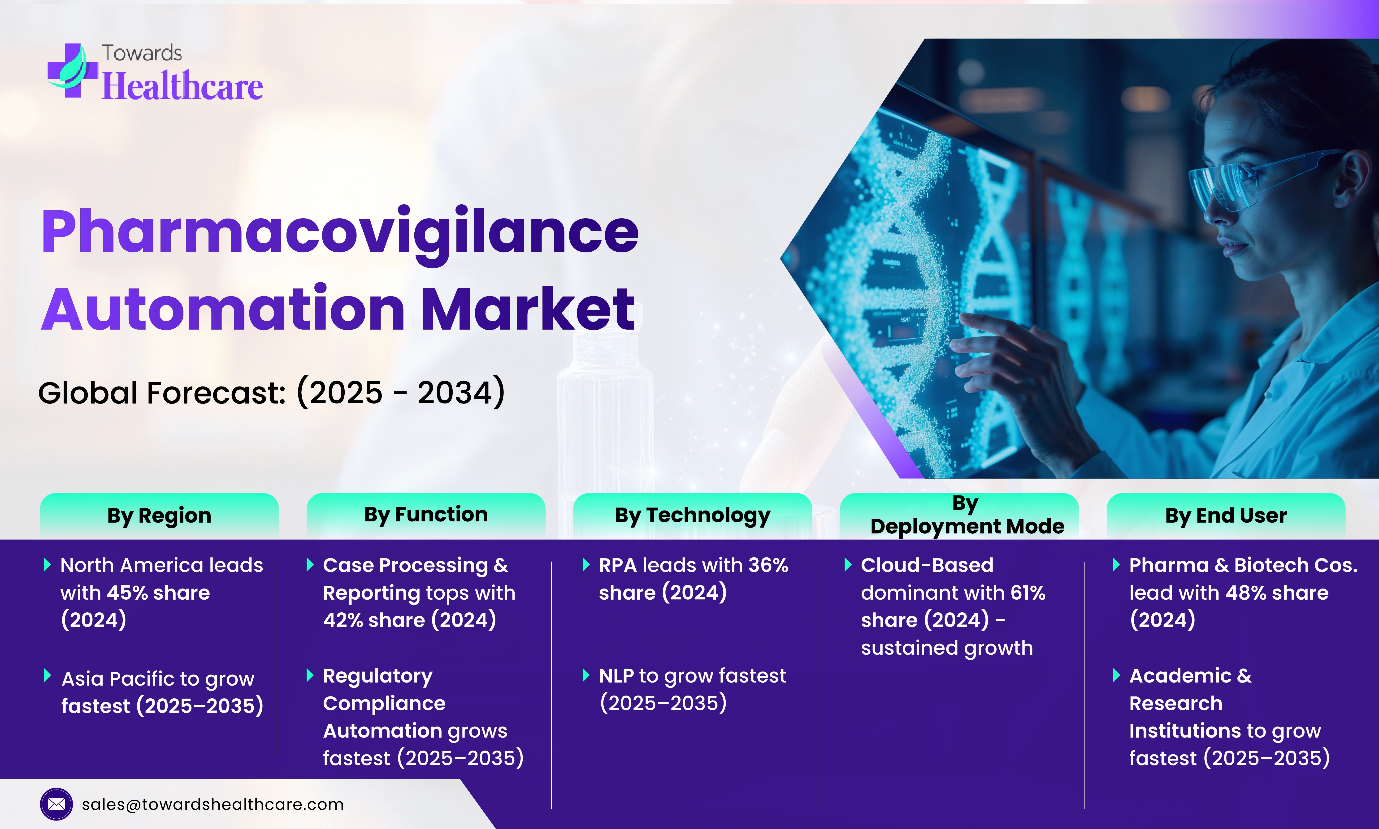
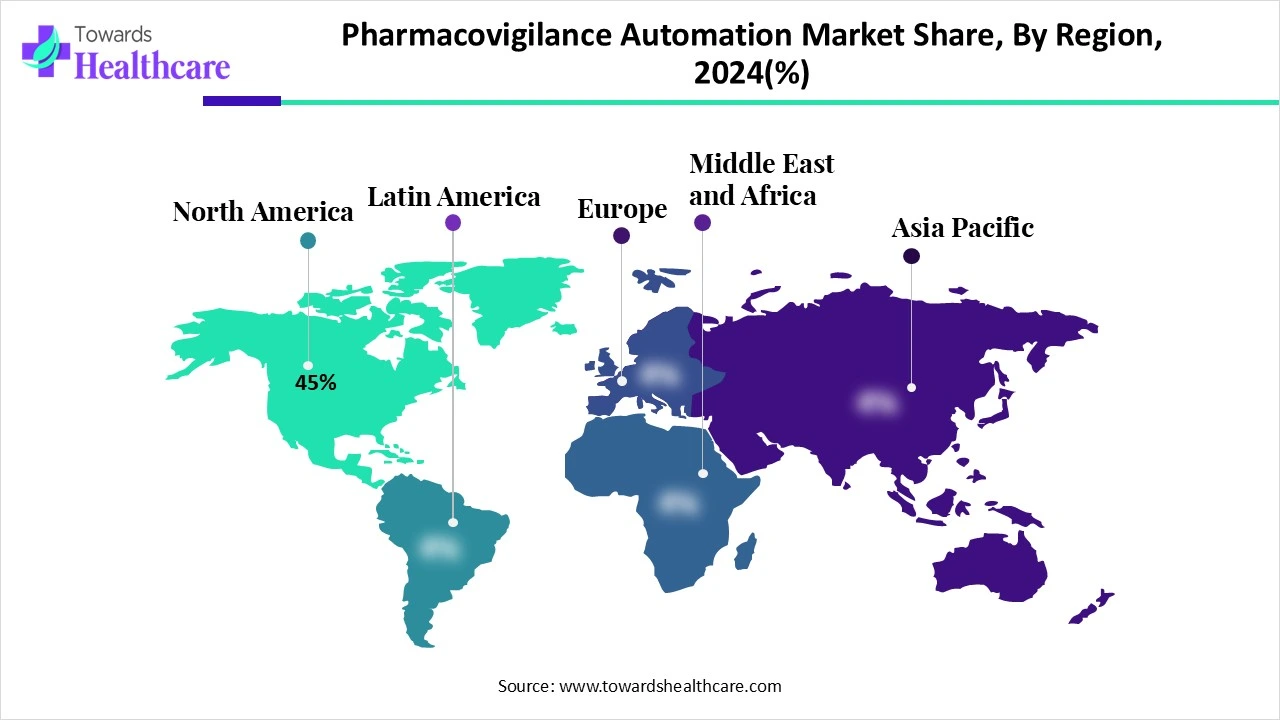
- North America is dominant in the pharmacovigilance automation market in 2024 with a 45% share.
- Asia Pacific is estimated to grow at the fastest CAGR from 2025 to 2035.
- By function, the case processing & reporting segment for the largest market revenue in 2024, with a 42% share.
- By function, the regulatory compliance automation segment is estimated to fastest-growing over the forecast period, 2025 to 2035.
- By technology, the robotic process automation (RPA) segment is dominant in the market in 2024 with a 36% share.
- By technology, the natural language processing (NLP) segment is expected to register the fastest growth over the forecast period, 2025 to 2035.
- By deployment mode, the cloud-based segment is dominant in the pharmacovigilance automation market in 2024, and it is expected to sustain the position during the forecast period with a 61% share.
- By end user, the pharmaceutical & biotechnology companies segment is dominant in the market in 2024 with a 48% share.
- By end user, the academic & research institutions segment is expected to register the fastest growth over the forecast period, 2025 to 2035.
Market Overview & Potential
What is the Growth Potential Responsible for the Growth of the Pharmacovigilance Automation Market?
The growth of the market is driven by the growing demand and increasing drug development, fueled by the expanding pharmaceutical industry's demand for safety monitoring systems, which in turn fuels the growth of the market. The rising adverse drug reactions monitoring due to increasing chronic diseases demands effective pharmacovigilance practices. Other key growth drivers are the regulatory stringency, technological advancement to automate the task and improve the accuracy, and to streamline the assessment boosts the growth and expansion of the market.
What are the Growing Trends Associated with the Pharmacovigilance Automation Market?
AI and Machine Learning:
- AI and ML are increasingly utilized to automate various tasks such as identifying adverse events, extracting data from unstructured sources like social media, and monitoring literature.
Automated Case Processing:
- Organizations are swiftly implementing automation for case handling, including intake, triage, and analysis, resulting in quicker reporting and greater efficiency.
Big Data Analytics:
- Pharmacovigilance is employing big data analytics to detect patterns and correlations within large datasets, supporting signal detection and risk management.
Real-World Evidence (RWE):
- The use of real-world data information collected outside clinical trials is on the rise, with automation playing a vital role in managing and analyzing this data effectively.
Remote Work and Collaboration:
- The pandemic has sped up remote work adoption, and automation tools are facilitating improved collaboration and communication among pharmacovigilance teams.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What is the Growing Challenge in the Pharmacovigilance Automation Market?
The key challenge that limits the growth of the Asia Pacific bioplastics market is the lack of sufficient funding for pharmacovigilance activities, resulting in limited implementation of advanced automation solutions, which limits the growth of the market. Other key challenge the is the shortage of skilled professionals who are experts in pharmacovigilance and automation technologies, which is a challenge and hinders the growth and expansion of the market.
Regional Analysis
How did North America Dominate the Pharmacovigilance Automation Market in 2024?
North America dominated the pharmacovigilance automation market share by 45% in 2024, with a 45% share, driven by early adoption of automation in healthcare, including PV, which effectively manages inventory, orders, and tracking, ensuring proper medicine delivery and reducing errors. The FDA’s Adverse Event Reporting System (FAERS) supports market growth by tracking adverse drug reactions and medication errors.
Advanced technology infrastructure, stringent FDA regulations, and growing AI adoption promote pharmacovigilance automation. Emphasis on real-time data analytics, electronic health records integration, and robust drug safety frameworks drives automated adverse event reporting and signal detection to enhance patient safety and regulatory compliance.
Canada’s focus on digital health transformation, Health Canada’s regulatory support, and investment in AI-powered pharmacovigilance tools promote automation. Collaboration among healthcare providers, researchers, and tech companies accelerates real-world data utilization, improving adverse event monitoring and drug safety through streamlined, automated reporting systems.
What Made Asia Pacific Significantly Grow in the Pharmacovigilance Automation Market in 2024?
The Asia Pacific region is projected to experience the fastest growth during the forecast period. Its rise is fueled by rapid product launches in healthcare, increasing consumer expectations, and the expanding use of digital health technology. Ongoing healthcare transformation, supported by policies, infrastructure development, and public-private partnerships, positions APAC to become a global leader in digital health, thus propelling market growth of market growth.
China’s expanding pharmaceutical industry, government initiatives for digital health, and adoption of big data and AI technologies drive pharmacovigilance automation. The National Medical Products Administration encourages automation to enhance drug safety surveillance, leveraging vast healthcare data to improve adverse event detection and regulatory oversight.
India’s growing pharmaceutical sector, government policies supporting digital health, and increased AI integration promote pharmacovigilance automation. The Central Drugs Standard Control Organization encourages automated adverse event reporting to enhance patient safety. Collaborative efforts between government, industry, and academia drive the adoption of technology for efficient drug safety monitoring.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Segmental Insights
By function
The case processing & reporting segment for the largest pharmacovigilance automation market revenue in 2024, with a 42% share. It provides data for investigating adverse effects, enabling the identification of new safety concerns and occasional assessment of the profit-to-challenge ratio for pharmaceutical use. It facilitates data exchange among stakeholders like patients, healthcare professionals, and authorities by sharing large volumes of safety information. Case reports play a key role in detecting new safety signals through the description of rare or delayed adverse effects that might not be observed in clinical trials.
The regulatory compliance automation segment is estimated to fastest-growing over the forecast period, 2025 to 2035. Automation in regulatory compliance ensures that medications and healthcare devices meet strict standards of quality, safety, and efficacy before market release. Agencies such as the FDA, EMA, and WHO enforce guidelines like GMP, GCP, and GDP throughout all stages of development and distribution. Following these guidelines is crucial for maintaining public trust and ensuring success in the medical industry.
By technology
The robotic process automation (RPA) segment is dominant in the pharmacovigilance automation market in 2024 with a 36% share. Robotic process automation (RPA) enhances operational efficiency by streamlining workflows, reducing risks, and minimizing delays and rework, which lowers operational costs. RPA offers privacy and security benefits, helps pharmaceutical companies reduce human errors, and improves data accuracy to support better decision-making. In pharmacovigilance, RPA is especially valuable for faster adverse effect response times.
The natural language processing (NLP) segment is expected to register the fastest growth over the forecast period, 2025 to 2035. NLP has become essential in pharmacovigilance, a field focused on monitoring and preventing drug-related adverse effects. It efficiently manages vast amounts of unstructured data such as patient records and social media posts, which provide real-world insights into drug effects. NLP aids in regulatory compliance and accelerates reporting to health authorities.
By deployment mode
The cloud-based segment is dominant in the pharmacovigilance automation market in 2024, and it is expected to sustain the position during the forecast period with a 61% share. Cloud solutions deliver robust security features like data encryption, secure access protocols, and regular backups. As an emerging tool, cloud-based systems in pharmacovigilance support seamless data integration by providing internet-based storage, computing services, analytics, and software, thus enhancing efficiency.
By end user,
The pharmaceutical & biotechnology companies segment is dominant in the pharmacovigilance automation market in 2024 with a 48% share. Pharmacovigilance companies share core goals with government agencies: protecting patients from harm by detecting hidden drug complexities early, identifying risk factors, debunking false safety signals, and addressing benefit challenges. PV raises awareness among healthcare professionals and patients regarding contraindications, indications, doses, administration routes, and adverse effects.
The academic & research institutions segment is expected to register the fastest growth over the forecast period, 2025 to 2035. Pharmacovigilance also hosts leading research platforms that improve patient care through informed prescribing and ensure government compliance. These collaborations with top industry players further fuel market growth.
Elevate your healthcare strategy with Towards Healthcare. Enhance efficiency and drive better outcomes schedule a call today: https://www.towardshealthcare.com/schedule-meeting
Recent Developments in the Pharmacovigilance Automation Market
- In July 2025, Tata Consultancy Services (TCS) was recognized as a Leader in Everest Group’s PEAK Matrix for Pharmacovigilance (PV) Operations services. The report emphasizes TCS’s extensive pharmacovigilance offerings, including device complaint management, medical device product quality issues, medical device reporting, and post-market surveillance as key strengths. TCS’s flagship ADD safety platform is notably highlighted for its touchless processing of individual case safety reports (ICSRs), featuring integrated quality control, smart signal detection, literature surveillance, and safety report generation.
- In September 2024, Truliant Consulting releases a white paper titled AI and Automation in Pharmacovigilance: Transformative Trends and Future Outlook. This paper results from an open survey aimed at pharmaceutical companies to explore their adoption of artificial intelligence (AI) within their Pharmacovigilance (PV) frameworks.
Top Companies and Their Contributions to the Market
| Company | Contributions & Offerings |
| Oracle Corporation | Provides cloud-based safety and pharmacovigilance solutions, integrating AI for automated case processing and compliance management. |
| Veeva Systems Inc. | Offers cloud software tailored for life sciences, including pharmacovigilance modules that streamline safety data management and reporting. |
| ArisGlobal | Develops end-to-end drug safety and pharmacovigilance platforms with automation, AI, and regulatory compliance features. |
| IQVIA | Combines real-world data analytics with automation to enhance signal detection, adverse event reporting, and clinical safety workflows. |
| Cognizant Technology Solutions | Delivers IT and BPO services focusing on automated pharmacovigilance processes, leveraging AI and machine learning for data accuracy. |
| Accenture | Provides digital transformation and AI-driven pharmacovigilance automation services to optimize safety data workflows globally. |
| Genpact | Specializes in AI-powered pharmacovigilance automation, improving adverse event case processing and regulatory reporting efficiency. |
| Tata Consultancy Services (TCS) | Offers integrated technology solutions automating safety data capture, analysis, and regulatory submissions in pharmacovigilance. |
| Parexel International Corporation | Provides clinical research and safety automation services, enhancing pharmacovigilance with AI-enabled case management and reporting tools. |
| ICON plc | Focuses on clinical research and drug safety automation, utilizing advanced analytics and automated adverse event monitoring systems. |
Pharmacovigilance Automation Market Key Players List

- Oracle Corporation
- Veeva Systems Inc.
- ArisGlobal
- IQVIA
- Cognizant Technology Solutions
- Accenture
- Genpact
- Tata Consultancy Services (TCS)
- Parexel International Corporation
- ICON plc
- Capgemini SE
- PharmaLex GmbH
- Indegene Pvt Ltd
- BioClinica (part of ERT)
- Infosys Limited
- HCL Technologies
- Ennov
- Tech Mahindra
- Zifo RnD Solutions
- NNIT A/S
Browse More Insights of Towards Healthcare: Global Pharmaceutical & Compounding Market Outlook (2024–2034)
The pharmaceutical and healthcare packaging sectors are undergoing notable transformation, supported by rising demand for personalized treatments, automation, and regulatory compliance. Here’s a breakdown of key market trajectories:
- GCC Compounding Pharmacy Market
The GCC compounding pharmacy market is witnessing a strong upward trend. Valued at USD 16.39 million in 2024, it is expected to reach approximately USD 40.26 million by 2034, growing at a CAGR of 9.27%. This growth is driven by the increasing need for tailored medications across the Gulf region. - Global Non-Sterile Compounding Pharmacy Market
The non-sterile compounding pharmacy market was estimated at USD 5.97 billion in 2024 and is set to almost double by 2034, reaching around USD 12.11 billion. With a CAGR of 7.34%, the market reflects expanding demand for dosage flexibility and patient-specific drug formulations. - Global Pharmacy Automation Market
The pharmacy automation market is advancing rapidly as healthcare systems adopt technologies to streamline dispensing and improve safety. Starting at USD 6.35 billion in 2024, the market is projected to reach USD 16.65 billion by 2034, growing at a CAGR of 10.12%. - Global Compounding Pharmacy Market
The global compounding pharmacy market stood at USD 13.19 billion in 2023 and is projected to climb to USD 22.91 billion by 2034. This steady growth, at a CAGR of 5.15%, is propelled by rising chronic disease prevalence and the need for customized drug therapies. - Global Vials Used in Compounding Pharmacy Market
The vials used in compounding pharmacy market is set for strong revenue growth over the forecast period. The market is expanding in response to higher demand for safe and precise medication containers, especially in sterile compounding. - Global Biotechnology & Pharmaceutical Services Market
The biotechnology and pharmaceutical services market is projected to grow from USD 76.51 billion in 2024 to around USD 130.56 billion by 2034, at a CAGR of 5.48%. The market benefits from increased outsourcing, R&D intensity, and regulatory support. - Global Biopharmaceuticals CRO Market
The biopharmaceuticals CRO (Contract Research Organization) market is on a robust growth path. With rising R&D and a push for cost efficiency, the market is expected to generate significant revenue gains through 2034. - Global Pharmaceutical Spray Drying Market
The pharmaceutical spray drying market is valued at USD 2.37 billion in 2024 and forecast to nearly double, reaching USD 4.93 billion by 2034. A CAGR of 7.67% reflects the increased use of this technology for enhancing bioavailability in drug formulations. - Global Ready-to-Use Pharmaceutical Packaging Market
The ready-to-use pharmaceutical packaging market was valued at USD 10.4 billion in 2024 and is anticipated to reach USD 20.97 billion by 2034. Growing at a CAGR of 7.24%, this market is shaped by demand for efficiency, sterility, and compliance. - Global Pharmaceutical Secondary Packaging Market
The pharmaceutical secondary packaging market is estimated at USD 43.11 billion in 2024 and is expected to grow to USD 69.45 billion by 2034. With a CAGR of 4.94%, this segment is critical for safety labeling, tracking, and brand protection.
Segments Covered in The Report
By Function
- Case Processing & Reporting
- Case Intake
- Medical Review
- Data Entry & Coding
- Signal Detection & Management
- Literature Screening
- Regulatory Compliance Automation
- Risk Management & Benefit-Risk Evaluation
- Report Submission & Distribution
- Quality Control & Reconciliation
By Technology
- Robotic Process Automation (RPA)
- Artificial Intelligence (AI) & Machine Learning
- Natural Language Processing (NLP)
- Cloud-Based PV Platforms
- Rules-Based Automation Tools
- Intelligent Automation
By Deployment Mode
- On-Premises
- Cloud-Based
By End User
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations (CROs)
- Pharmacovigilance Service Providers
- Regulatory Agencies
- Academic & Research Institutions
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
To invest in our premium strategic solution and customized market report options, click here: https://www.towardshealthcare.com/price/5824
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Gain access to the latest insights and statistics in the healthcare industry by subscribing to our Annual Membership. Stay updated on healthcare industry segmentation with detailed reports, market trends, and expert analysis tailored to your needs. Stay ahead of the curve with valuable resources and strategic recommendations. Join today to unlock a wealth of knowledge and opportunities in the dynamic world of healthcare: Get a Subscription
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire
Find us on social platforms: LinkedIn | Twitter | Instagram
