Austin, Aug. 05, 2025 (GLOBE NEWSWIRE) -- Laboratory Developed Tests Market Size & Trends
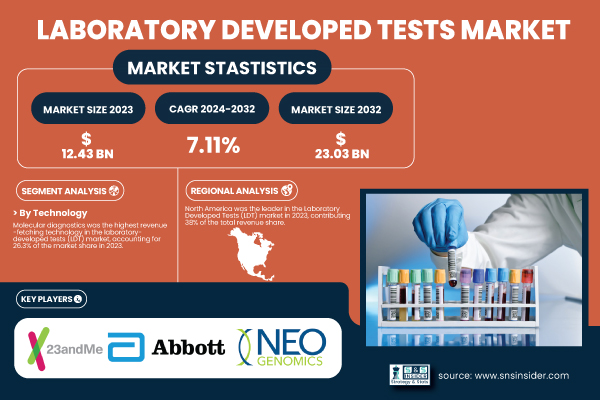
According to a new report by SNS Insider, the Laboratory Developed Tests Market was valued at USD 12.43 billion in 2023 and is projected to reach USD 23.03 billion by 2032, at a CAGR of 7.11% during the forecast period.
In the U.S., the laboratory-developed tests market size was USD 3.57 billion in 2023 and will grow at a CAGR of 5.5% to reach USD 5.62 billion by 2032.
Get Free Sample Report of the Laboratory Developed Tests Market: https://www.snsinsider.com/sample-request/6185
Laboratory Developed Tests (LDTs) are essential diagnostics designed, manufactured, and used within a single laboratory, particularly important for specialized testing such as genetic screening, oncology, rare diseases, and infectious diseases. The market is expanding rapidly due to the increasing emphasis on personalized medicine, next-generation sequencing (NGS), and companion diagnostics. Rising incidence of chronic diseases, technological innovations, and the need for rapid and cost-effective testing are contributing to the market’s robust growth globally.
Despite some regulatory uncertainties, particularly in the U.S., demand for LDTs is accelerating as healthcare providers increasingly adopt precision-based diagnostics for better patient outcomes.
Major Players Analysis Listed in this Report are:
- Quest Diagnostics Incorporated – OncoVantage, Cardio IQ, NeuromeDx
- 23andMe, Inc. – Genetic Health Risk Reports, Carrier Status Reports
- Abbott – Vysis LDT FISH Probes, RealTime PCR LDTs
- Guardant Health – Guardant360 LDT, GuardantOMNI LDT
- NeoGenomics Laboratories – NeoTYPE Cancer Profiles, NGS Fusion Testing
- Siemens Healthineers AG – Atellica Solution LDTs, ADVIA Chemistry LDTs
- QIAGEN – QIAseq NGS Panels, TheraScreen Companion Diagnostics
- Illumina, Inc. – TruSight Oncology 500, VeriSeq NIPT Solution
- F. Hoffmann-La Roche Ltd. – FoundationOne CDx, AVENIO ctDNA Assays
- BioReference Laboratories, Inc. – GenPath Oncology LDTs, ClariTest Prenatal Screening
Segment Analysis
By Technology
Molecular diagnostics was the leading technology in the LDT market in 2023, with a global share of 26.3% of market revenue. The segment is predominantly leading due to its high usage for the identification of genetic mutation, infectious diseases, and cancers using procedures including the PCR, NGS, and microarrays. Hospitals and reference labs depend on molecular diagnostics, which are high precision and high throughput.
By Application
Oncology was the most revenue-generating application in 2023 and accounted for 23.01% of the total market. Its ascendancy is driven by the need for early and accurate profiling of tumor tissue for the use of targeted therapies and minimal residual disease monitoring. LDTs now play indispensable roles in cancer diagnostics, particularly in solid tumors and hematological malignancies.
Regional Analysis
North America was the highest contributor to the global laboratory-developed tests market in 2023, accounting for 38% share of revenue. This leadership is attributed to the region’s well-established healthcare industry and availability of high-complexity CLIA-certified labs and R&D investments. In oncology and infectious disease, the U.S. continues to be the hub of innovation around LDTs owing to a large universe of patients and the existence of top diagnostic developers and academic centers.
Asia Pacific is experiencing the fastest growth in the laboratory-developed tests market, with access to molecular diagnostics being expanded, greater healthcare spending, and government programs to advance genomic research. As investing in global diagnostic infrastructure and precision medicine facilities accelerates, China, India, South Korea, etc., present huge opportunities for LDT providers.
For a Personalized Briefing with Our Industry Analysts, Connect Now: https://www.snsinsider.com/request-analyst/6185
Recent Developments
- Feb 2025 – Molecular Instruments, Inc. launched HCR Pro RNA” ISH technology on its LDTs in partnership with Yale Dermatology to help the company differentiate psoriasis and eczema with their four-biomarker panel.
- Apr 2024 – NeoGenomics announced the introduction of a novel LDT for multi‐gene fusion detection in hematologic malignancies and support of targeted therapy selection.
- Jan 2024 – Invitae launched a new LDT for rapid exome sequencing for the most critically ill paediatric patients, with a focus on reducing diagnostic turnaround times for neonates in the ICU.
Laboratory Developed Tests Market Segmentation
By Technology
- Immunoassays
- Hematology and Coagulation
- Molecular Diagnostics
- Microbiology
- Clinical Chemistry
- Histology/Cytology
- Flow Cytometry
- Mass Spectroscopy
- Others
By Application
- Oncology
- Companion Diagnostics
- Genomics Sequencing & Other
- Genetic Disorders/Inherited Disease
- Infectious & Parasitic Diseases
- Immunology
- Endocrine
- Nutritional & Metabolic Disease
- Cardiology
- Mental/Behavioral Disorder
- Pediatrics-specific Testing
- Hematology/General Blood Testing
- Bodily Fluid Analysis
- Toxicology
- Other Diseases
Laboratory Developed Tests Market Report Scope
| Report Attributes | Details |
| Market Size in 2023 | US$ 12.43 billion |
| Market Size by 2032 | US$ 23.03 billion |
| CAGR | CAGR of 7.11% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Regional Analysis | North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, Poland, Turkey, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Rest of Latin America) |
| Key Growth Drivers |
|
Suggested Unique USP Sections for Client Proposal
To differentiate the report and add premium analytical depth, consider including the following unique USP sections:
- LDT Regulatory Evolution Tracker- An in-depth timeline and comparative analysis of evolving global regulatory frameworks (e.g., VALID Act in the U.S.), assessing potential implications for market entry and scalability.
- Clinical Utility Mapping by Application- A detailed matrix evaluating the clinical impact, test adoption rate, and reimbursement coverage across major LDT applications (e.g., oncology vs infectious disease vs genetic testing).
- Adoption Lifecycle Curve by Facility Type- Insight into adoption trends across academic hospitals, reference labs, private diagnostics, and research institutes, highlighting readiness levels and investment strategies.
- Innovation Pipeline Dashboard- A graphical dashboard showcasing high-impact LDTs in the pipeline by test type, biomarker focus, and anticipated launch window, based on real-time announcements and patent activity.
- Reimbursement Landscape Snapshot- Detailed analysis of payer coverage across key markets (U.S., Germany, Japan, etc.), identifying LDTs with favorable reimbursement trends and those at risk.
- Tech-Convergence Case Studies- Real-world examples of how AI, machine learning, and cloud-based platforms are being integrated into LDT workflows to improve test accuracy, reduce TAT, and enable remote diagnostics.
Buy the Full Laboratory Developed Tests Market Report (Single-User License) Now: https://www.snsinsider.com/checkout/6185
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
