Austin, May 06, 2025 (GLOBE NEWSWIRE) -- Clinical Trial Supplies Market Size & Growth Analysis:
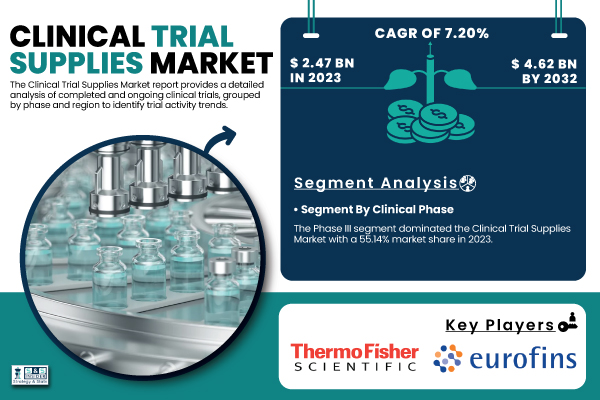
According to the latest report by SNS Insider, the global Clinical Trial Supplies Market, valued at USD 2.47 billion in 2023, is anticipated to reach USD 4.62 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 7.20% from 2024 to 2032.
Rising R&D expenditure by pharmaceutical and biotechnology firms, the global count of clinical trials, and the increased focus on precision and personalized medicine help to explain this strong development. Furthermore, the industry is seeing a move towards distributed and virtual trials, requiring agile and responsive supply chain models.
Get a Sample Report of Clinical Trial Supplies Market@ https://www.snsinsider.com/sample-request/6026
The U.S. Clinical Trial Supplies Market value of USD 0.81 billion in 2023 is expected to rise to USD 1.47 billion by 2032, with a CAGR of 6.92%. A strong national position of the country is fueled by the high volume of clinical trials carried out, modern healthcare infrastructure, a good regulatory environment, and the presence of big pharmaceutical sponsors. Under its Real-Time Oncology Review (RTOR) program, initiatives by the U.S. FDA modernize clinical trial procedures, simplifying the drug development process and increasing demand for dependable and adaptable supply chain solutions.
Key Clinical Trial Supplies Companies Profiled in the Report
- Thermo Fisher Scientific (Clinical Ancillary Supplies, Comparator Drugs)
- VWR International (Laboratory Chemicals, Protective Clothing)
- Grifols (Plasma-Derived Medicines, In Vitro Diagnostic Products)
- Catalent Pharma Solutions (Clinical Supply Services, Biologics Development)
- Parexel International (Clinical Trial Management, Regulatory Consulting)
- PRA Health Sciences (Clinical Research Services, Data Solutions)
- ICON plc (Clinical Development, Laboratory Services)
- PPD (Pharmaceutical Product Development) (Clinical Trial Management, Laboratory Services)
- Almac Group (Clinical Trial Supply, Analytical Services)
- Covance Inc. (Clinical Trial Testing, Drug Development Services)
- Medpace Holdings, Inc. (Clinical Trial Management, Medical Device Development)
- Eurofins Scientific (Bioanalytical Testing, Genomic Services)
- WuXi AppTec (Laboratory Testing, Clinical Development)
- Charles River Laboratories (Preclinical Services, Clinical Support)
- Biocair International (Specialist Logistics, Temperature-Controlled Transport)
- Movianto (Healthcare Logistics, Warehousing Solutions)
- Fisher Clinical Services (Clinical Supply Chain Management, Packaging Solutions)
- Sharp Clinical Services (Clinical Trial Packaging, Distribution Services)
- PCI Pharma Services (Clinical Trial Supply, Packaging Solutions)
- Clinigen Group (Clinical Supplies Management, Comparator Sourcing)
Clinical Trial Supplies Market Report Scope
| Report Attributes | Details |
| Market Size in 2023 | US$ 2.47 billion |
| Market Size by 2032 | US$ 4.62 billion |
| CAGR | CAGR of 7.20% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Key Regional Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East]), Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia Rest of Latin America) |
Segmentation Analysis
By Clinical Phase
The Phase III clinical trials segment dominated the market in 2023, comprising 55.14% revenue share. Often comprising thousands of participants across multiple sites and countries, phase III trials are the most thorough and resource-intensive phase. The great scope and high cost of these studies generate a strong need for effective supply chain management, packaging, labeling, comparator sourcing, and distribution solutions. Clinical trial supplies are essential to guarantee the continuous operation of these frequently longer-running trials involving several stakeholders, including CROs, regulatory agencies, and logistics partners. Expected to keep this category dominating during the projected period are the ongoing development of new medicines and the necessity to demonstrate safety and efficacy at scale.
By Product & Service
The Supply Chain Management segment emerged as the leading category, comprising 62.22% of the clinical trial supplies market in 2023. The growing complexity of worldwide clinical trials, especially in distributed and hybrid models requiring customized supply chain methods, is attributed to this dominance. Essential for guaranteeing compliance and reducing delays, services in this area include planning, forecasting, warehousing, comparative medicine purchasing, and logistics tracking. Integrated supply chain services get even more value from the move toward temperature-sensitive biologics and cell & gene treatments. Investment in end-to-end supply chain solutions keeps increasing as sponsors concentrate on risk reduction and operational effectiveness, hence driving development in this market.
By Therapeutic Use
The oncology therapeutic applications dominated the clinical trial supplies market, comprising a 65.32% market share in 2023. Particularly immunotherapies, biologics, and precision medicine, cancer treatments account for the most of the clinical pipelines worldwide. Usually involving extended terms, intensive patient monitoring, and sophisticated protocol designs, oncology trials demand specific supply solutions. Cold chain management, comparative medicine sourcing, and timely delivery across several countries are among logistics' needs. Additionally, strict regulatory requirements for oncology studies make real-time inventory tracking and protocol compliance vital. As pharmaceutical investment in cancer therapies continues to rise, this segment is expected to remain the largest contributor to clinical trial supply demand.
By End Use
With 65.22% of the total revenue, the pharmaceutical segment held the largest market share in 2023. Leading pharmaceutical corporations are outsourcing clinical trial supply operations to outside service providers and CROs more and more. Pharmaceutical companies are using modern supply chain systems to handle multi-region studies, keep protocol compliance, and improve cost-efficiencies as drug development becomes more global and complicated. Moreover, the growing attention on biologics and specialized medicines has resulted in more investment in temperature-controlled supply chain systems. Along with the necessity of quick trial releases and adaptive designs, this tendency helps the segment to remain leading.
For A Detailed Briefing Session with Our Team of Analysts, Connect with Us Now@ https://www.snsinsider.com/request-analyst/6026
Clinical Trial Supplies Market Segmentation
By Clinical Phase
- Phase I
- Phase II
- Phase III
- Others
By Product & Service
- Manufacturing
- Storage & Distribution
- Cold Chain Distribution
- Non-Cold Chain Distribution
- Supply Chain Management
By Therapeutic Use
- Oncology
- CNS Diseases
- Cardiovascular Diseases
- Infectious Diseases
- Metabolic Disorders
Others
By End Use
- Pharmaceuticals
- Biologics
- Medical device
- Others
Regional Analysis
With 42.81% of the global revenue, North America dominated the clinical trial supplies market in 2023. Strong pharmaceutical sector presence in the area, several active clinical studies, and advanced regulatory support systems help to define this leadership. Key players in the U.S. and Canada include Thermo Fisher, Parexel, and Catalent, which run strong supply networks. Adoption of digital trial tools and the FDA's modernization programs helps to keep the regional ecosystem strengthened.
Rising clinical trial activity in nations such as China, India, Japan, and South Korea is predicted to cause the Asia-Pacific area to grow fastest throughout the projection period. Globally minded pharmaceutical businesses are drawn to favourable legislative developments, reduced running costs, and great patient availability. Governments in the area are also funding harmonizing regulatory paths and bettering of the logistical infrastructure, which will probably increase demand for the provision of clinical trial tools.
Recent Developments
- Catalent Inc. added a new distribution center in Japan in January 2024 to assist with rising trials in the Asia-Pacific area, therefore broadening its clinical supply network.
- In February 2024, Thermo Fisher Scientific announced a collaboration with Medidata to maximize digital supply chain systems for hybrid and virtual trials.
Buy a Single-User PDF of Clinical Trial Supplies Market Analysis & Outlook Report 2024-2032@ https://www.snsinsider.com/checkout/6026
Table of Contents – Major Key Points
1. Introduction
2. Executive Summary
3. Research Methodology
4. Market Dynamics Impact Analysis
5. Statistical Insights and Trends Reporting
6. Competitive Landscape
7. Clinical Trial Supplies Market by Clinical Phase
8. Clinical Trial Supplies Market by Product & Service
9. Clinical Trial Supplies Market by Therapeutic Use
10. Clinical Trial Supplies Market by End Use
11. Regional Analysis
12. Company Profiles
13. Use Cases and Best Practices
14. Conclusion
Related Reports
Virtual Clinical Trials Market Report
In Silico Clinical Trials Market Report
Clinical Trial Investigative Site Network Market Report
Clinical Trial Management System Market Report
Clinical Trial Imaging Market Report
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
