Pune, March 25, 2025 (GLOBE NEWSWIRE) -- Influenza Diagnostics Market Size & Growth Analysis:
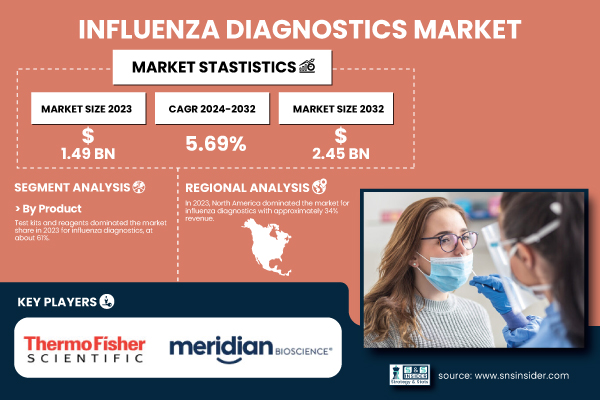
According to SNS Insider, the global Influenza Diagnostics Market was valued at USD 1.49 billion in 2023 and is projected to reach USD 2.45 billion by 2032, growing at a CAGR of 5.69% from 2024 to 2032. The market’s expansion is primarily driven by the increasing prevalence of influenza, advancements in rapid diagnostic testing, and growing awareness of early detection and disease management.
Get a Sample Report of Influenza Diagnostics Market@ https://www.snsinsider.com/sample-request/4668
Market analysis
The increasing burden of influenza coupled with the growing demand for early and precise diagnosis is driving the uptake of new diagnostic tools and techniques. In 2023, the US accounted for an 11% share of the global influenza diagnostics market and held 35% of the North American market due to the country's sophisticated healthcare infrastructure, high influenza prevalence, and substantial governmental support for diagnostic innovations. In 2023, the U.S. Department of Health and Human Services (HHS) announced a USD 1.2 billion investment to strengthen influenza surveillance and diagnostics capabilities nationwide, which is, in turn, boosting the market growth.
The increase in the prevalence of influenza cases globally is one of the major factors driving the market. Seasonal influenza epidemics cause 3 to 5 million cases of severe disease and 290,000 to 650,000 respiratory deaths each year, according to the World Health Organization (WHO). Increasing investments in influenza testing, and the growing importance of precise respiratory virus diagnostics This further incorporates developments in diagnostics including the introduction of rapid molecular assays and point-of-care (POC) testing systems to improve the accuracy and availability of influenza detection. The market growth is further supported by government and healthcare organizations emphasizing influenza surveillance and preparedness.
Major Players Analysis Listed in this Report are:
- 3M Company: 3M Molecular Diagnostics System
- Abbott Laboratories: BinaxNOW Influenza A & B Card
- Becton, Dickinson and Company (BD): BD Veritor System for Rapid Detection of Flu A & B
- Meridian Bioscience, Inc.: ImmunoCard STAT! Flu A & B
- Quidel Corporation: Sofia Influenza A+B FIA
- F. Hoffmann-La Roche Ltd: cobas Influenza A/B Test
- SA Scientific Ltd: Rapid Influenza Test Kit
- SEKISUI Diagnostics: QuickVue Influenza A+B Test
- Thermo Fisher Scientific, Inc.: TaqMan Influenza A/B PCR Test
- Hologic, Inc.: Panther Fusion Influenza A/B Assay
- Siemens (Germany): CLINITEK Status+ for Influenza A/B
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China): MINDRAY Rapid Test for Influenza
- Koninklijke Philips N.V. (Netherlands): Philips Respironics Influenza A/B Test
- NeuroLogica Corp. (U.S.): CereTom for Infectious Disease Detection
- Shimadzu Medical (India) Pvt. Ltd. (Japan): PCR Detection Systems for Influenz
- GENERAL ELECTRIC (U.S.): GE Healthcare Diagnostic Imaging for Influenza
- Quest Diagnostics Incorporated (U.S.): Quest Influenza A & B Test
- Sysmex India Pvt. Ltd. (Japan): Sysmex Flu Test
- Hitachi, Ltd. (Japan): Hitachi Medical Systems for Infectious Disease Testing
- Canon Inc. (Japan): Canon Medical Influenza Testing Solutions
- FUJIFILM Holdings Corporation (U.K.): Fujifilm Rapid Influenza Test
- GenMark Diagnostics, Inc. (U.S.): ePlex Respiratory Pathogen Panel
Influenza Diagnostics Market Report Scope
| Report Attributes | Details |
| Market Size in 2023 | US$ 1.49 billion |
| Market Size by 2032 | US$ 2.45 billion |
| CAGR | CAGR of 5.69% From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Data | 2020-2022 |
| Key Regional Coverage | North America (US, Canada, Mexico), Europe (Eastern Europe [Poland, Romania, Hungary, Turkey, Rest of Eastern Europe] Western Europe] Germany, France, UK, Italy, Spain, Netherlands, Switzerland, Austria, Rest of Western Europe]), Asia Pacific (China, India, Japan, South Korea, Vietnam, Singapore, Australia, Rest of Asia Pacific), Middle East & Africa (Middle East [UAE, Egypt, Saudi Arabia, Qatar, Rest of Middle East]), Africa [Nigeria, South Africa, Rest of Africa], Latin America (Brazil, Argentina, Colombia Rest of Latin America) |
Segmentation Analysis
By Product
In 2023, the test kits and reagents led the influenza diagnostics market with around 61% share of total revenue. Analyzes of influenza viruses are performed using a variety of methodologies requiring test kits and reagents. This product remains in high demand due to its wide applications in hospitals, diagnostic laboratories, and clinics. Moreover, multiplex assay development is contributing to the increased adoption of both test kits and reagents, as these can help measure multiple respiratory pathogens in a single process. As the demand for effective and quick influenza diagnostics continues to surge, prominent players in the field are concentrating on introducing state-of-the-art and easy-to-use test kits.
By Test
The traditional flu testing diagnostics segment led the market in 2023, holding around 65% of the revenue share. Conventional diagnostic tests, including rapid influenza diagnostic tests (RIDTs) and viral culture, are still frequently employed despite the advantages of these methods because of their relatively low cost and ease of use. In particular, rapid immunodiagnostic tests (RIDTs) have gained prominence, owing to their capability of providing results in 15-30 min to point-of-care, without specialized laboratory equipment. However, the market is slowly transitioning to molecular diagnostics including RT-PCR and NAATs, which provide improved sensitivity and specificity. However, the traditional methods are still widespread, particularly in resource-limited environments where sophisticated diagnostics infrastructure is unavailable.
By End-Use
In 2023, the largest share of the influenza diagnostics market was contributed by hospitals and clinics segment, at 46% of total revenue. Influenza testing is usually performed at hospitals and clinics where sophisticated instruments exist and patients are always influx. The growing emphasis on early diagnosis and treatment of influenza as well as its rising prevalence has propelled the demand for influenza diagnostics in these settings. With the rapid advancement of point-of-care testing devices in hospitals and clinics, influenza diagnostics became more efficient, allowing for faster decision-making and improving patient outcomes.
Need Any Customization Research on Influenza Diagnostics Market, Enquire Now@ https://www.snsinsider.com/enquiry/4668
Key Influenza Diagnostics Market Segments
By Product
- Test Kit and Reagents
- Instruments
- Others
By End-use
- Hospitals and clinic
- Diagnostic Labs
- Others
By Test
- Traditional Influenza Diagnostic Tests
- Rapid Influenza Diagnostic Tests (RIDTs)
- Direct Fluorescent Antibody (DFA) Tests
- Viral Culture
- Serological Assays
- Molecular Influenza Diagnostic Tests
- Reverse Transcription Polymerase Chain Reaction (RT-PCR)
- Isothermal Nucleic Acid Amplification Tests (INAAT)
- Loop-Mediated Isothermal-based Amplification Assays
- Transcription Mediated Isothermal-based Amplification Assays
Regional Analysis
North America accounted for approximately 34% share of revenue in the global influenza diagnostics market in 2023. Advanced healthcare infrastructure, high healthcare spending, and a strong emphasis on influenza surveillance and preparedness contribute to the region's leadership. The United States had the largest market in North America, attributed to the prevalence of influenza and the key market players in the region. The Centers for Disease Control and Prevention (CDC) estimated that the U.S. saw 35 million influenza cases, 380,000 hospitalizations, and 28,000 deaths from the 2022-2023 flu season. In the United States, the strong influenza surveillance system of the CDC combined with widespread vaccination campaigns has also propelled the demand for influenza diagnostics.
Europe held a significant share of the market, driven by stringent healthcare regulations and increasing investments in diagnostic technologies. Advanced testing for influenza is most established in Germany, France, and the UK, given government initiatives and robust healthcare systems. The Asia-Pacific region is projected to be the fastest-growing region during the forecast period, which can be attributed to the growing healthcare investments, developing diagnostic infrastructure, and increasing awareness regarding influenza prevention and control. Because the burden of influenza is increasing, countries such as China, India, and Japan are concentrating on increasing their diagnostic capacity.
Recent Developments
- In 2023, Abbott Laboratories released a new rapid molecular test for influenza that delivers results in 15 minutes with high accuracy. Advancements like this are set to transform point-of-care influenza diagnostics.
- Roche Diagnostics announced FDA approval in January 2024 of its cobas Liat System, an analyte-specific reagents test for real-time PCR-based detection of influenza with high sensitivity and specificity, provided within 4 hours of sample receipt.
Buy a Single-User PDF of Influenza Diagnostics Market Analysis & Outlook Report 2024-2032@ https://www.snsinsider.com/checkout/4668
Table of Contents – Major Key Points
1. Introduction
2. Executive Summary
3. Research Methodology
4. Market Dynamics Impact Analysis
5. Statistical Insights and Trends Reporting
5.1 Incidence and Prevalence (2023)
5.2 Prescription Trends, (2023), by Region
5.3 Drug Volume: Production and usage volumes of pharmaceuticals.
5.4 Healthcare Spending: Expenditure data by government, insurers, and out-of-pocket by patients.
6. Competitive Landscape
7. Influenza Diagnostics Market by Product
8. Influenza Diagnostics Market by End-use
9. Influenza Diagnostics Market by Test
10. Regional Analysis
11. Company Profiles
12. Use Cases and Best Practices
13. Conclusion
Access Complete Report Details of Influenza Diagnostics Market Analysis & Outlook 2024-2032@ https://www.snsinsider.com/reports/influenza-diagnostics-market-4668
[For more information or need any customization research mail us at info@snsinsider.com]
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
