YUCAIPA, Calif., Jan. 27, 2010 (GLOBE NEWSWIRE) -- Ingen Technologies, Inc. (Pink Sheets:IGNT), a leading manufacturer of respiratory medical devices, recently announced today that it has approved an insertion order with COPD Foundation's new magazine that targets 20,000-30,000 lung specialists that includes physicians, respiratory therapists, nurse practitioners, and other lung specialists.
A photo accompanying this release is available at http://www.globenewswire.com/newsroom/prs/?pkgid=7025
The company already has a 12 month contract with the COPD Foundation's COPD Digest magazine that is distributed once each quarter to over 200,000 subscribers. The new magazine will be released next month and includes articles and submissions by a variety of academics and clinicians, and will be focused on articles of interest to those treating patients with pulmonary diseases. These lung specialists represent 15,000,000 patients with COPD and various lung disorders in the USA.
"We look forward to working with Mitch Ruttner of the COPD Foundation and supporting the efficacy of their program. Mr. Ruttner has also been very proactive with our Oxyview product line, as we continue to direct a strong focus in the commercial hospital markets to sell our products. We continue to see an increase with hospital orders for bulk purchases, and this is an indicator that our Oxyview products are being recognized by hospitals. We are very appreciative for all of the support Ingen continues to receive from credible sources and shareholders," stated Scott R. Sand, Chief Executive Officer and Chairman of the Board.
http://www.copdfoundation.org/programs/copd_digest
http://www.oxyviewnasalcannula.com/AboutUs.html
About Ingen:
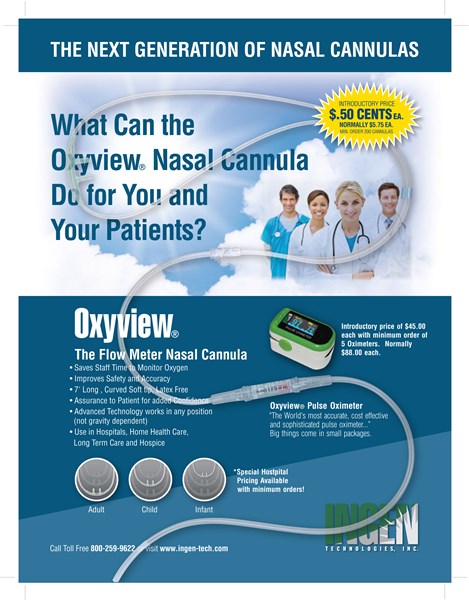
Ingen is an established medical device manufacturer with an emerging new medical product line for the respiratory market worth an estimated $4 Billion in the U.S., and $8 billion globally. The company introduced Oxyview into the respiratory market in late 2007 after securing U.S. and Foreign Patents and a successful registration with the Food & Drug Administration. The company has domestic and global distribution with manufacture representative organizations, and OEM partners. In 2009 the Oxyview Nasal Cannula was introduced as the world's first oxygen cannula with an in-line pneumatic oxygen flow meter. In 2010 the company introduced the Oxyview Pulse Oximeter. The Oxyview product line is available to the home care markets, commercial medical markets, aviation, automotive, and government sources. The company is licensed with the Department of Health and Human Services, and manufactures its products in the State of California. With approximately 32 million U.S. patients with Chronic Obstructive Pulmonary Disease (COPD), and 600 million patients worldwide, Ingen Technologies is now the largest manufacturer of in-line gravity-independent oxygen flow meters.
The Ingen Technologies, Inc. logo is available at http://www.globenewswire.com/newsroom/prs/?pkgid=2472
Safe Harbor for Forward-Looking Statements: This news release includes forward-looking statements. While these statements are made to convey to the public the company's progress, business opportunities and growth prospects, readers are cautioned that such forward-looking statements represent management's opinion. Whereas management believes such representations to be true and accurate based on information and data available to the company at this time, actual results may differ materially from those described. The company's operations and business prospects are always subject to risk and uncertainties. Important factors that may cause actual results to differ are and will be set forth in the company's periodic filings with the U.S. Securities and Exchange Commission.
The photo is also available at Newscom, www.newscom.com, and via AP PhotoExpress.